International Journal of Veterinary Science and Research
Prevalence and Multidrug Resistance Profiles of Escherichia coli in Dairy Farms
DVM, MSc VPH, Haramaya University, Ethiopia
Author and article information
Cite this as
Netsanet Tadesse T (2020) Prevalence and Multidrug Resistance Profiles of Escherichia coli in Dairy Farms. Int J Vet Sci Res. 2020; 6(2): 142-147. Available from: 10.17352/ijvsr.000065
Copyright License
© 2020 Netsanet Tadesse T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Calf diarrhoea, commonly occur in cattle herds, impacting on the economic viability of cattle operations. A total of 72 calves under 6-months of age, 38 exotic breeds (50% Holstein-Friesian X 25% Jersey X 25% Ogaden) from a dairy farm and 34 local breeds from the Veterinary Clinic, were studied. The secondary data on calf management and impact of calf diarrhoea were collected from the dairy farm, veterinary clinic and subsistence smallholder’s dairy farms. Faeces samples were collected from all calves in a sterile container, kept in an ice-cold condition and cultured in the time period of 24 hours. Nearly 49% of the faecal samples were positive for Escherichia coli. E. coli O157 Latex Plate Agglutination test revealed 4.2% and 8.3% prevalence in exotic and local breeds, respectively. Herd size, age group, weaning age were found to be significantly associated (p< 0.05) with the occurrence of E. coli. Antimicrobial susceptibility was tested using agar disk diffusion method, and it was found that E. coli possessed resistance to ampicillin, erythromycin, vancomycin and penicillin. In contrast, amoxicillin, sulphamethoxazole, streptomycin, kanamycin, ciprofloxacin and tetracycline were effective against E. coli.
Calf-hood diseases result a significant economic consequences on the viability of cattle operations such as the direct costs of calf losses, the cost for therapeutic measures, surveillance activities to monitor the prevalence of the pathogen, long-term effects on performance and future productivity [1-3].
Failure in passive immunity transfer and overwhelming pathogen exposure are the main precipitating factors for calf diarrhoea. Mechanisms for the occurrence of diarrhoea in calves include hypersecretion of ions and water into the bowl increased osmotic pressure from maldigestion (malabsorption disease caused by damage to enterocytes), increased mucosal permeability due to inflammation and the less important mechanism is an alteration of intestinal motility [4].
Escherichia coli is mainly an enteric bacteria in animals and can also survive in the environment, e.g., dairy products and faecal contaminated materials [5-7]. it is one of the most important foodborne illnesses pathogens that commonly hosted in Dairy cattle leads to a serious infection in animals and humans [8]. Normally, colisepticemia (bacteria invade the systemic circulation and internal organs) and enteric colibacillosis (bacteria are localized in the lumen and mucosal surface of the small intestine) are the common E. coli associated diseases. Serotypes of E. coli possesses unique attributes of virulence that discriminate serotypes of E. coli [9]. E coli serotypes that able to adhere on to the wall of the small intestine have K99 fimbrial antigen. They attached, with their fimbriae, to the wall of the intestine and produce enterotoxins, stimulating excessive intestinal secretions leading into severe diarrhoea mostly in calves of less than one week of age [9,10]. Thus, fimbrial antigens (K99) or enterotoxins are indicators for E. coli derived diarrhoea in dairy calves [11].
Antimicrobial agents are commonly used for disease control and as a growth promoter in the commercial farms [12]. However, the inappropriate usage of antibiotics leads to the extensive spread of antibiotic-resistant organisms [13,14]. Antibiotic-resistant bacteria causes nearly a 10 million mortality deaths and associated risks in the world [15,16].
Altogether, Calf diarrhea is one of the most common disorder hindering the economy and productivity in dairy farms [17,18]. Zoonotic infectious diseases account for an estimated 60% of all human infectious diseases [19] and Calf Diarrhea accounts for approximately 75% of the mortality of dairy calves under three weeks of age [20]. Neonatal calf diarrhea causes the illness and mortality due to various pathogens including E. coli [21] which is influenced by farm location, herd size and management [17]. Thus, the prevalence of E. coli in diarrheic and non-diarrheic calves, antimicrobial susceptibility and the other possible risk factors were studied.
Materials and methods
Study area and sampling
This study was conducted at Haramaya district (42o30´ E, 9o26´ N), eastern Hararghe, Ethiopia. It has an elevation of 2047 m.a.s.l., receives a mean annual rainfall of 780 mm, and mean minimum and maximum temperatures of 8.3 oC and 23.4 oC, respectively.
Calves under 6-months of age were sampled (West, 1995) and grouped into 0-3 and 3- to 6-months. Faecal samples were taken from diarrhoea and non-diarrheic calves in the district, specifically, from Haramaya University Dairy Farm (HUDF), the Veterinary Clinic of the district and subsistence smallholder’s dairy farms. In HUDF, unlike others, the faecal sample was collected from all calves. The ear tags (in HUDF) and codes were used during faecal sampling and downstream studies. The secondary data including calf management and effects of diarrhoea were collected.
The sample size was determined based on [22] and previous reports (Holeta dairy farm, Ethiopia), 38% expected prevalence in crosses and Borena breeds [23]:
Where: n = required sample; Pexp = Expected prevalence; and d2 = Desired absolute precision
Accordingly, at 95% confidence interval and 0.05 absolute precision, the total number of animals planned to be included in the study were 185. However, a total of 72 calves were used for this study because of unavailability of required number of calves under six-month age in the HUDF, Veterinary Clinic and subsistence smallholder’s dairy farms.
Study design and sample collection
A cross-sectional type of study was conducted to determine the possible etiologic agents of calf diarrhoea. The questionnaire survey was also conducted to assess overall farm management skills and practices, which was helpful to determine the potential risk factors, e.g., age, sex, breed, herd size and colostrum feeding.
Faecal samples were collected directly from the rectum of the calf with a sterile plastic glove. About ten grams of faeces were collected from rectum and put in a sterile universal bottle. During sample collection, data including the date, ear tag or assigned code, age, sex, breed, type of colostrum feeding, weaning age, herd size, were recorded in a separate sheet, while the relevant piece of information and ear tag or code of the calf was labelled on the universal bottle. Finally, samples were kept at an ice-cold condition and transported to the Microbiology Laboratory College of Veterinary Medicine, Haramaya University. The samples were cultured within 24 hours of sample collection.
E. coli isolation procedures
E. coli Isolation Procedures: five-gram faeces was inoculated into 45 ml BPW at 37 oC for 18 hours and subculture was made at 24 hours to selective plate media, Eosin methylene blue agar(EMB agar) (OXOID, Germany), and MacConkey agar (OXOID, Germany). Suspicious colonies were further sub-cultured in nutrient media for biochemical tests. The suspected pure colonies from nutrient agar were inoculated in MRVP broth (for MR and VP tests), in tryptophan media (for Indole test), TSIA slant (OXOID, Germany), citrate slant (OXOID, Germany), LIA (OXOID, Germany) and urea broths (OXOID, Germany). Lysine decarboxylase broth positive, MR positive, VP negative, Indole positive, yellow (acid) slant, yellow (acid) butt, H2S negative and gas produced in TSI, and urease negative were identified as E. coli [11].
E. coli o157 latex plate agglutination test
The test was performed following Thermo Scientific Oxford Microbiology (2001-2020) procedures: one drop of the test latex was dispensed onto a circle on the reaction card and placed close to the edge of the circle. A pasteur pipette drop of saline was added to the circle. A loop pick off a portion of the colony was obtained and tested. The test latex was mixed together and spread to cover the reaction area using the loop then the loop was flamed. The card was rocked for 1 minute in a circular motion, observing for agglutination. A portion of the colony was tested when the agglutination with the test reagent was occurred. The positive results were observed in the form of precipitation unlike the case in negative samples.
Antimicrobial susceptibility test
The antibiotic susceptibility test was carried out using agar disk diffusion method [11]. The antibiotic disks used were ampicillin (10 μg), amoxicillin (25 μg), sulphamethoxazole (25 μg), streptomycin (10 μg), erythromycin (15μg), tetracycline (30 μg), kanamycin (30 μg), ciprofloxacin (5 μg), vancomycin (30 μg), and penicillin (10 units). The pure bacterial suspension was made from a nutrient agar in a BPW. The suspension was standardized using a 0.5 McFarland standard.
About 4-5 well-isolated colonies (pure bacterial colonies) were selected from a non-selective agar plate (nutrient agar was used). The top of the colonies was touched and the growth was transferred to a test tube containing 4-5 ml of Trypton Soya broth (OXOID, Germany). The bacterial suspension was standardized with a 0.5 McFarland turbidity standard. The bacterial suspension was then inoculated on to a Mueller-Hinton agar plate using a sterile swab, after five minutes of absorption, the antimicrobial impregnated discs were placed at the equidistant position and incubated. After 18 hours of incubation at 37 °C, the diameter of the zone of inhibition was measured using a millimeter scale for each antimicrobial disc on the undersurface of the plate. The zone size around each antimicrobial disk was interpreted as susceptible, intermediate or resistant according to modified NCCLS criteria [11].
Data analysis
Descriptive statistics were used to summarize recorded data. Association of potential risk factors with the occurrence of E coli was also conducted using SPSS (version 17.0) statistical package. Chi-square (χ) test and p-value at 95 % confidence interval were used to determine associations. A χ2 test at the specified degree of freedom was interpreted as the presence of significant association if the calculated χ2 value was greater than the χ2 value (at 95 % confidence interval). Moreover, a p-value of less than 0.05 (p< 0.05) was indicted as statistically significant. The logistic regression model was used to evaluate the odds ratio. The odds ratio was calculated to see the degree of association between the different risk factors and bacterial infection rates and interpreted as significantly associated by using the p-value and 95 % confidence interval for the odds ratio.
Results
Calf morbidity and mortality
Calves with 1- to 6-months age found in the HUDF, Veterinary Clinic of the district and subsistence smallholder’s dairy farms were considered for this study. HUDF possessed a total of 244 exotic cattle during the study period, of which, 38 were calves under 6-months age. In all, 72 calves were examined of which 38 were exotic (16 male and 22 female) and 34 were local (17 male and 17 female) breeds.
Weaning age
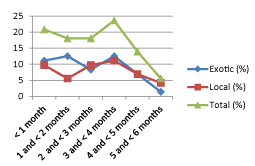
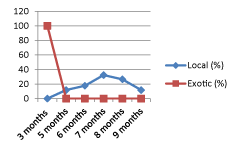
The exotic breeds weaned at the earlier stage (3-months) compared with the local breeds raged from 3- to 9-months (Figures 1,2).
Secondary data about morbidity and mortality could not be found from HUDF, but informally collected data showed that there were two to three deaths of calves per one year mainly because of calf scour and injury during parturition.
Feeding management
In HUDF, feeding starts with colostrum soon after birth and involves feeding of solid feeds to weaned calves. The calves were allowed to suckle. Liquid feeds were not provided to pre-weaned calves. Alfalfa and silage were common feeds available to weaned calves. Whereas, feeding involves colostrum and the mixture of fenugreek, water, and salt soon after and late after birth in the subsistence smallholder’s dairy farms, Solid feeds such as large and small cereals straw, and maize seedlings were also provided for weaned calves. The calves in smallholder’s dairy farms were also allowed to suckle.
Prevalence of E. coli
It was found that 45.8% (33) samples were positive for the analytical E. coli isolation procedures while 54.2% (39) were negative for E. coli. A higher prevalence of E. coli (51.6%) infection was observed in male calves, followed by female calves (41.0%), however, this difference is not significantly (p > 0.05) different Table 1.
χ2 =12.347, df = 1, p-value = 0.000
Exotic calves (73.7%) were less susceptible to E. coli infection compared with the local breeds (32.4 %), which was statistically (p < 0.05) significant (χ2 =12.347, df = 1).
The odds ratio was also calculated to see the degree of association between breed and prevalence of E. coli infection (Table 2).
According to the odds ratio, the odds (likelihood) of disease in local breeds was almost 6 times higher than that of the exotic breeds (OR = 5.855) and it was statistically significant (p < 0.05) (Table 2).
E. coli infection was significantly (p < 0.05) different in different herd sizes (Table 2). The highest prevalence of E. coli (100.0%) infection was noticed in farms having a herd size of 5, followed by 4 animals per herd having a prevalence of 77.8 %, then herd size of 3, having a 70.0 % prevalence of E. coli infection. According to the odds ratio, the odds (likelihood) of disease in local breed particularly with 5 calve (herd size) is nearly 3 times higher than the exotic breeds (OR = 2.8), which was statistically significant (p < 0.05).
E. coli infection was not significantly (p < 0.05) different in the start of colostrum feeding. A higher prevalence of E. coli (64.7%) infection was recorded in calves that fed colostrum late after birth (6 to 8 hours), followed by calves feed colostrum soon after birth having a prevalence of (40 %).
E. coli infection was significantly (p < 0.05) different in different weaning ages. A higher prevalence (81.8%) of E. coli was recorded in calves weaned at 7-months followed by calves weaned at 8-months (77.8%) and 9-months (75.0%).
E. coli infection was significantly (p < 0.05) different in different age groups. A higher (65.5%) prevalence of E. coli infection was found in calves between 3- to 6-months, while comparatively lower (32.6 %) prevalence of E. coli was observed in calves between 0- to 3-months old.
In the analysis of different risk factors with the occurrence of E. coli infection, the majority of the factors were found to be significantly (p < 0.05) associated.
According to the odds ratio, the odds (likelihood ) of disease in 0- to 3-months age group is nearly 4 times higher than that of the 3- to 6-month age group (OR = 3.936), and it was statistically (p < 0.05) different Table 2).
E. Coli O 157 latex plate agglutination test
E. coli was isolated from 45.83 % (33/72) of the total number of faecal samples. These E. coli isolates were subjected to serological identification using E. coli O 157 Latex Plate Agglutination. Accordingly, 4.17% and 8.33 % prevalence in exotic and local breeds, respectively, was observed.
Antimicrobial susceptibility test
It is not uncommon that drugs are different in their efficacy. Thus, 33 samples (positive for E. coli from 72 faecal samples) were tested for antimicrobial susceptibility (Table 3).
Discussion
Calf morbidity and mortality
The age of the calf is the important factor in calf morbidity and mortality for various reasons: a newborn calf has poorly developed defense mechanism, normal flora is not well established and unlike to newborn of primates, they are born with no circulating antibodies to combat infection [24]. In HUDF, there were 38 exotic breed calves of which nearly 71% of calves were below 4-months (Figure 1). Mostly the morbidity statistics of the dairy calf are not available, when available are not as reliable as those in mortality because they depend on the producers’ diagnosis, amount of time spent observing the animal, degree of illness expressed by the animal, and the tendency of producers not to record every illness events [25].
The secondary data about morbidity and mortality showed 2 to 3 deaths of calves per year mainly because of calf scours and injury while getting birth. Considering 3 calves death in a year and a total number of calves under 6-month (38 calves), the crude death rate was estimated 7.89 %. The calf mortality rates in Ethiopia ranges from 7 to 25% in pre-weaned calves [23]. Other reports in Ethiopia also revealed 15% in the first month and 8% 1- to 3-months [26]. The crude death rate (7.89%) in HUDF was comparatively low which could be achieved via the conducive environment, good management practices and herd size. Herd size by itself is not a biological effect on the calf health; rather, it may be a measurement of other factors, e.g., time allocation for calves’ management. Proper management [27] and smaller heard size [25] significantly reduce calf mortality. Records on calves morbidity and mortality in the study area was not adequate, thus proper data documentation is needed to avail reliable information.
Weaning age
The weaning age of local breeds started at 3-months, the proportion was increased until 7-months and then declined until 9–months whereas exotic breeds were weaned between 3- to 5-months (Figure 2). In all, the weaning ages in the study area were at acceptable range for current and upcoming performance of calves. Basically expected that calves weaned in 3-to 5-months are healthy and better in body weight. This may be associated with the concept that the better performance of late weaned calves in transition from milk to solid feed compared with early weaned calves [28] that can be because of a gradual shift of microbiota in digestive system [29].
Feeding management
Feed and feeding methods are important risk factors in morbidity and mortality of dairy calves. In HUDF, feeding starts with colostrum soon after birth and involves feeding solid feeds, mainly alfalfa and silage. Suckling was also supplemented with colostrum feeding to increase the volume. This is most likely the reason for the reduction of calf mortality in HUDF. The passive transfer immunity can be improved when suckling is supplemented with bottle feeding [30]. Calf mortality is significantly higher in calves that got inadequate colostrum in a24 hours of birth or receive late after birth [5,25,31]. An elevated plane of nutrition in the very beginning month leads into a greater productivity and growth. This is because colostrum and transition milk comprise a plenty of bioactive molecules that favors gut development and microbiota as well [32]. However, a transition from liquid pre-weaned feed to solid weaned calf feed needs to done carefully to reduce dietary stress and reduce morbidity [5,33].
Prevalence of E. coli in fecal samples
Many researchers confirmed the prevalence of E. coli in diarrheic calves which is mostly associated with the age and low colostrum feeding [34-36]. In this study, E. coli was isolated from fecal samples. Differences in proportion of infected local and exotic breeds were observed (Table 1). The prevalence of E. coli was higher (31.94%) in local breeds than the exotic breeds (13.89%); calculated based on the positive samples of E. coli of each breed and total fecal samples (Table 1). Moreover, E. coli o157 showed 4.17 % prevalence in exotic calves and 8.33 % prevalence in local breeds.
The calculated X2 value (0.792) was less than the tabulated value (3.841) at 1 degree of freedom which showed that the prevalence of E. coli on male (51.5%) and female calves (41.0%) was statistically not significant at 5% significance level, indicating the prevalence of E. coli did not depend on sex difference.
The prevalence of E. coli was significant (P< 0.05) between breeds that was greater prevalence on local (67.6 %) than exotic (26.3 %) breeds. Regression analysis result revealed the risk of E. coli infection in local breeds was nearly 5.9 times higher than the exotic breeds which was statistically significant (P< 0.05) (Table 2).
The prevalence of E. coli was influenced by herd size. The calculated X2 value (15.512) was greater than the tabulated value (9.488) at 4 degree of freedom, revealing significantly (P< 0.05) different prevalence of E. coli on different herd sizes. Furthermore, regression analysis (significant at P< 0.05) showed the risk of E. coli infection in larger herd size is about 2.8 times higher than the lower herd size. Generally, from regression analysis it was found that the risk of E. coli infection in age was about 6.3 times higher than the breed and herd size, and is statistically (P< 0.05) significant.
The prevalence of E. coli was significantly (P< 0.05) influenced by the time of colostrum feeding after birth. The calculated X2 value (3.193) was less than the tabulated value (3.841) at 1 degree of freedom at 5% significance level, however greater than tabular value (2.706) at 1 % significance level. There was more prevalence of E. coli (64.7 %) on calves those start suckling comparatively late after birth than calves start colostrum feeding soon after birth.
The prevalence of E. coli was significantly (P< 0.05) influenced by the time of weaning age and age differences. The calculated X2 value (17.044) and (7.578) was greater than the tabulated value (11.070) and (3.841) at 5 and 1 degrees of freedom of weaning age and age differences, respectively. The regression analysis also revealed the risk of E. coli infection in 0-to 3-months age group was nearly 3.9 times higher than the 3- to 6-months age group.
Antimicrobial susceptibility test
It is crucial to timely treat diseased calves and wise to select drugs for successful treatment. In this study it was found that E. coli possessed resistance to ampicillin, erythromycin, vancomycin and penicillin. In contrast, amoxicillin, sulphamethoxazole, streptomycin, kanamycin, ciprofloxacin and tetracycline were effective against E. coli.
Conclusions
The HUDF had effective farm management practices with crude death rate (7.9%). The prevalence of E. coli was higher (31.94 %) in local breeds than the exotic breeds (13.89 %). The prevalence of E. coli was influenced by breed type, herd size, time of colostrum feeding, weaning age and age difference, but not by sex difference. E. coli possessed resistance to ampicillin, erythromycin, vancomycin and penicillin. In contrast, drugs such as amoxicillin, sulphamethoxazole, streptomycin, kanamycin, ciprofloxacin and tetracycline were effective against E. coli. Thus, this finding can contribute to the future antimicrobial resistance monitoring in Ethiopia.
- Chang Q, Wang W, Regev-Yochay G, Lipsitch M, Hanage WP (2015) Antibiotics in agriculture and the risk to human health: How worried should we be? Evolutionary Applications 8: 240–247. Link: https://bit.ly/3jfiLvP
- Ghoddusi A, Nayeri Fasaei B, Zahraei Salehi T, Akbarein H (2019) Prevalence and characterization of multidrug resistance and variant Salmonella genomic island 1 in Salmonella isolates from cattle, poultry and humans in Iran. Zoonoses and Public Health 66: 587–596. Link: https://bit.ly/2FEUjq4
- Sharma C, Rokana N, Chandra M, Singh BP, Gulhane RD, et al. (2018) Antimicrobial resistance: Its surveillance, impact, and alternative management strategies in dairy animals. Front Vet Sci 4. Link: https://bit.ly/31jUWNs
- Hunt E (1993) Diarrhoeal disease of neonatal ruminants. In H. J.L (Ed.), Current Veterinary Therapy, Food Animal Practice. (3rd ed.). Philadelphia: W.B Sounders Company.
- Mohammed AN, Abdel-Latef GK, Abdel-Azeem NM, El-Dakhly KM (2016) Ecological study on antimicrobial-resistant zoonotic bacteria transmitted by flies in cattle farms. Parasitology Research 115: 3889–3896. Link: https://bit.ly/359UrXn
- Narvaez-Bravo C, Toufeer M, Weese SJ, Diarra MS, Deckert AE, et al. (2016) Prevalence of methicillin-resistant Staphylococcus aureus in Canadian commercial pork processing plants. J Appl Microbiol 120: 770–780. Link: https://bit.ly/3lWJxLm
- Rodriguez-Rivera LD, Cummings KJ, Loneragan GH, Rankin SC, et al. (2016) Salmonella Prevalence and Antimicrobial Susceptibility among Dairy Farm Environmental Samples Collected in Texas. Foodborne Pathog Dis 13: 205-211. Link: https://bit.ly/34akoXF
- Halimi HA, Seifi HA, Rad M (2014) Bovine salmonellosis in Northeast of Iran: Frequency, genetic fingerprinting and antimicrobial resistance patterns of Salmonella spp. Asian Pac J Trop Biomed 4: 1-7. Link: https://bit.ly/2FG1het
- Acres SD (1985) Enterotoxigenic E. coli infection in newborn calves: A review. J Dairy Sci 68: 229-256. Link: https://bit.ly/35dOzw7
- Abraham G, Roeder PL, Zewdu R (1992) Agents associated with neonatal diarrhoea in Ethiopian dairy calves. Tropical Animal Health and Production 24: 74–80. Link: https://bit.ly/3dKwSZ5
- Quinn PJ (1994) Clinical Veterinary Microbiology. London: Wolf Publishing. Link: https://bit.ly/3o5Adqf
- Schlundt J, Aarestrup FM (2017) Commentary: Benefits and risks of antimicrobial use in food-producing animals. Frontiers in Microbiology 8. Link: https://bit.ly/3keByc7
- Li B, Webster TJ (2018) Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. Journal of Orthopaedic Research 36: 22–32. Link: https://bit.ly/2HeVf5p
- Murgia M, Bouchrif B, Timinouni M, Al-Qahtani A, Al-Ahdal MN, et al. (2015) Antibiotic resistance determinants and genetic analysis of Salmonella enterica isolated from food in Morocco. Int J Food Microbiol 215: 31–39. Link: https://bit.ly/31kH9pO
- Prestinaci F, Pezzotti P, Pantosti A (2015) Antimicrobial resistance: A global multifaceted phenomenon. Pathogens and Global Health 109: 309-318. Link: https://bit.ly/3o6AidB
- Tule A, Hassani U (2017) Colonization with Antibiotic-Resistant E. coli in Commensal Fecal Flora of Newborns. International Journal of Current Microbiology and Applied Sciences 6: 1623-1629. Link: https://bit.ly/37kiSUA
- Cho Y, Yoon KJ (2014) An overview of calf diarrhea - infectious etiology, diagnosis, and intervention. Journal of Veterinary Science 15: 1–17. Link: https://bit.ly/3mcrGjV
- El-Seedy FR, Abed AH, Yanni HA, Abd El-Rahman S. A. A. (2016). Prevalence of Salmonella and E. coli in neonatal diarrheic calves. Beni-Suef University Journal of Basic and Applied Sciences 5: 45–51. Link: https://bit.ly/37gUFi8
- Taylor LH, Latham SM, Woolhouse MEJ (2001) Risk factors for human disease emergence. Philosophical Transactions of the Royal Society B: Biological Sciences 356: 983–989. Link: https://bit.ly/3lXz02z
- Blowey R (1999) A Vetenary Book For Dairy Farmers (2nd ed). Farming press Ltd.
- Izzo MM, Kirkland PD, Mohler VL, Perkins NR, Gunn AA, et al. (2011) Prevalence of major enteric pathogens in Australian dairy calves with diarrhoea. Australian Veterinary Journal 89: 167–173. Link: https://bit.ly/37gUUd2
- Thrusfield M (2005) Veterinary epidemiology (3rd ed.). Blackwell Science, Ames, IA.
- Shiferaw Y, Yohannes A, Yilma Y, Gebrewold A, Gojjam Y (2002) Dairy husbandry and health management at holleta. Proceeding of the 16thconference of the Ethiopian veterinary association. Addis Ababa. Link: https://bit.ly/3o4s8Cw
- Bath DL, Dickinson FR (1985) Dairy Cattle: Problems, Practices.
- Bruning-Fann C, Kaneene JB (1992) Environmental and management risk factors associated with morbidity and mortality in perinatal and pre-weaning calves: a review from an epidemiological perspective. Veterinary Bulletin 62: 399-413. Link: https://bit.ly/35hLyeC
- Gryseels K, de Boodet G (1986) Integration of Crossbred cows (Boran and Freisian) on smallholder farms in Debre Zeit area of the Ethiopian highlands. Addis Ababa. Link: https://bit.ly/2T61g6L
- Radostis O, Heinrichs J (2001) Health and Production Management of Dairy Calves and Replacement Heifers. In R. O.M. (Ed.), Herd Health Food Animal Production medicine (3rd ed., pp. 333–395). Philadelphia: W.B. Saunders Company.
- Meale SJ, Leal LN, Martín-Tereso J, Steele MA (2015) Delayed weaning of Holstein bull calves fed an elevated plane of nutrition impacts feed intake, growth and potential markers of gastrointestinal development. Animal Feed Science and Technology 209: 268-273. Link: https://bit.ly/3o4suJm
- Meale SJ, Li SC, Azevedo P, Derakhshani H, DeVries TJ, et al. (2017) Weaning age influences the severity of gastrointestinal microbiome shifts in dairy calves. Scientific Reports 7: 198. https://doi.org/10.1038/s41598-017-00223-7
- Brignole TJ, Stott GH (1980) Effect of Suckling Followed by Bottle Feeding Colostrum on Immunoglobulin Absorption and Calf Survival. Journal of Dairy Science 63: 451-456. Link: https://bit.ly/3dEVH8I
- Wells SJ, Dargatz DA, Ott SL (1996) Factors associated with mortality to 21 days of life in dairy heifers in the United States. Preventive Veterinary Medicine 29: 9-19. Link: https://bit.ly/3m0VdNi
- Fischer AJ, Villot C, van Niekerk JK, Yohe TT, Renaud DL, et al. (2020) Corrigendum to “Invited Review: Nutritional regulation of gut function in dairy calves: From colostrum to weaning” (Appl. Anim. Sci. 35:498–510). Applied Animal Science 36: 133. Link: https://bit.ly/2T3uEKO
- Cray WC, Casey TA, Bosworth BT, Rasmussen MA (1998) Effect of dietary stress on fecal shedding of Escherichia coli O157:H7 in calves. Applied and Environmental Microbiology 64: 1975-1979. Link: https://bit.ly/3jbIrte
- Awosile BB, Smith BA (2017) Risk assessment modelling of fecal shedding caused by extended-spectrum cephalosporin-resistant Escherichia coli transmitted through waste milk fed to dairy pre-weaned calves. Journal of Dairy Science 100: 9667–9673. Link: https://bit.ly/2HiwPHG
- Disassa N, Sibhat B, Mengistu S, Muktar Y, Belina D (2017) Prevalence and Antimicrobial Susceptibility Pattern of E. coli O157:H7 Isolated from Traditionally Marketed Raw Cow Milk in and around Asosa Town, Western Ethiopia. Veterinary Medicine International 2017: 7581531. Link: https://bit.ly/31kIjSc
- Tadesse M, Abebe N, Gebru M, Husen N (2017) Major Causes and Risk Factors Associated with Calf Mortality in Small Scale Dairy Farms in Gondar Town , Ethiopia. Academic Journal of Animal Diseases 6: 67–74. Link: https://bit.ly/2T7mz80
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
