International Journal of Veterinary Science and Research
Detection and identification of blood-borne infections in goats in Nigeria using light microscopy and polymerase chain reaction
Anise N Happi1*, Deborah M. Buba2, Paul E Oluniyi3,4 and Kazeem Akano3,4
2Department of Veterinary Microbiology and Pathology, Faculty of Veterinary Medicine, University of Jos, Plateau State, Nigeria.
3Department of Biological Sciences, Redeemer’s University, Ede, Osun State, Nigeria
4African Centre of Excellence for Genomic of Infectious Diseases, Redeemer’s University, Ede Osun State, Nigeria
Cite this as
Happi AN, Buba DM, Oluniyi PE, Akano K (2020) Detection and identification of blood-borne infections in goats in Nigeria using light microscopy and polymerase chain reaction. Int J Vet Sci Res 6(2): 093-103. DOI: 10.17352/ijvsr.000060Haemoparasitisms in animals are known to impose substantial economic burdens on owners. In Nigeria, most laboratories utilize only Light Microscopy (LM) for their diagnosis. Hence there is a need to have an update assessment of haemoparasitism of goat in Nigeria using molecular investigation.
Using LM, blood samples from a total of 173 goats in Ibadan were screened for haemoparasites and haemocytopathological evaluation. Among them, 126 blood samples were randomly selected and PCR tested for the 18S rRNA gene of Babesia/Theileria spp (B/T) and the 16S rRNA gene of Trypanosoma brucei/Ehrlichia spp (A/E), while 91 samples were evaluated for the 16S RNA of To-GoTM (Hemoplasma). Relationship between the haematological changes and PCR results was evaluated.
The PCR detection rate was significantly and more than 4-fold higher compared to LM (61.5% and 14.5%, respectively; P < 0.0001) alone. A total of 150 animals were tested by both methods with 62.7% overall infection proportion. Comparison of LM and PCR analyses showed approximately 50% misdiagnosis of Hemoplasma spp by LM with 82.7% and 100% false negative for A/E and B/T recorded by LM, respectively. The haemoparasites detected by LM were Borrelia spp, Hemoplasma spp, Babesia spp, Trypanosoma brucei spp and, Trypanosoma brucei. A total of 34.1%, 23% and, 51.6% samples were positive for B/T, A/E and, Hemoplasma, respectively. Sequencing and phylogenetic analyses of some PCR products identified samples with high homology with Trypanosoma brucei ovis in the A/E group while the B/T were highly related to Theileria velifera. Sequencing of Hemoplasma positive samples identified Mycoplasma ovis, Mycoplasma wenyonii and Pseudomonas fluorescens. The haematological changes were unspecific to infection types and, showed no significant deviation between haemoparasite positive and negative animals.
The striking disparity between LM and PCR methods for haemoparasite detection and a larger repertoire of haemoparasites are presented. Borrelia spp, Mycoplasma wenyonii, Pseudomonas fluorescens and Theileria velifera are newly reported in goat with To-GoTM being the most prevalent in Ibadan. Studies geared towards pathogenicity investigation in the unusual host, their diversity and factors of transmission are envisaged for effective prevention and control.
Abbreviations
A/E: Trypanosoma brucei/Ehrlichia spp; ANOVA: Analysis of Variance; B/T: Babesia/Theileria spp; BLAST: Basic Local Alignment Search Tool; BLASTn: Nucleotide-nucleotide BLAST; Bo: Borellia spp; BSA: Bovine Serum Albumin; DNA: Deoxyribonucleic Acid; E. hystolytica: Entamoeba hystolytica; EDTA: Ethylenediaminetetraacetic Acid; H. spp: Hemoplasma spp; Haemo neg: Haemoparasite negative; Haemo pos: Haemoparasite positive; Hb: Haemoglobin; HM: To-GoTM/Hemoplasma; LM: Light Microscopy; MAFFT: Multiple Alignment using Fast Fourier Transform; NCBI: National Center for Biotechnology Information; PCR: Polymerase Chain Reaction; PCV: Packed Cell Volume; RNA: Ribonucleic Acid; rRNA: ribosomal RNA; RS: Red Sokoto; SPSS: Statistical Package for the Social Science; T. brucei: Trypanosoma brucei; T. cruzi: Trypanosoma cruzi; T. gondii: Trypanosoma gondii; T. spp: Trypanosoma spp; TBDs: Tick-Borne Diseases; TRBC: Total Red Blood Cell; Tryp: Trypanosome; TWBC: Total White Blood Cell; WAD: West African Dwarf
Introduction
Blood-borne parasitic diseases rank as the most important disease factor hindering small ruminants’ production in tropical and subtropical regions. In goats, these diseases are not well characterized, although small ruminant farming is the main livestock resource in some rural regions in Nigeria and Africa. The benefits derived from sheep and goats in the tropics are significantly below the expected. This is mainly due to reduced production associated to numerous factors, of which disease is the most important [1]. Goats in Sub-Saharan Africa are infected with a wide variety of parasites most importantly vector-borne haemoparasites [2,3]. Haemoparasitic infections have been reported in sheep in the Southwestern parts of Nigeria [4,5] and were attributed to suitable environmental conditions appropriate for the survival of vectors of the diseases [5]. Tick-Borne Diseases (TBDs) serve as limitation to livestock production in many developing countries of the world as they are responsible for increased morbidity and mortality resulting in reduced production of meat, milk and other livestock by-products [6-8]. Although economic losses caused by haemoparasitic diseases are known to be high worldwide [9,10], the direct losses caused by the haemoparasite infections are due to ill-health, premature slaughter, rejection of some body parts at meat inspection and death [11], while indirect losses could be attributed to lower production, quarantine measures, tick control, vaccination [12] and medications. Haemoparasitism creates limitation to improved productivity, animal health [13] and economic gain [14-17]. The continuous challenge of haemoparasitism and existence of carrier state in goat and sheep, are sources of threat to susceptible hosts. The continuous challenge is due to various factors such as the poor control measures of the vectors, resistance to the available drugs for the control of disease, very slow rate of production of new drugs due to the low interest, no vaccine available, minimal research funding in the area, lack of facilities in the research for diversity, rapid and accurate diagnostic test, drug target, parasite-host interaction for drug development and control of infection together with the very ticklish way of the parasites. In addition, misdiagnosis and or underdiagnoses of haemoparasitic infections may contribute to delay and unspecific treatment thus further contributing to the economic loss. However, despite the development and benefit of molecular methods of diagnosis, their adoption or implementation in the routine investigation of the haemoparasitic diseases have not improved in Nigeria as most Veterinary hospitals (public and private) and laboratories still rely on Light Microscopy (LM) diagnosis alone. Light microscopy diagnosis has been revealed to be poorly sensitive, insufficiently accurate and subjective. Underdiagnosis and misdiagnosis of haemoparasitic infections associated with the use of LM have been reported by various authors in Nigeria [18-20] and these have been attributed to various reasons ranging from the parasite similarity to some cell structures, other parasites, artifacts due to faulty processing of slides, level of parasitaemia, and the competency of the microscopist [19]. On the other hand, Polymerase Chain Reaction (PCR) provides a more accurate diagnosis owing to its higher sensitivity and specificity, and it is fast becoming an inevitable facility for routine diagnosis given the large repertoire of haemoparasitic diseases with the need for adequate/specific therapy. Thus, it is now known that the latter method is unavoidable, particularly in the tropical African practice as there is a strong need for its integration in routine screening for accurate and prompt diagnosis with consequent successful control and reduction in economic loss.
Diagnosis of some diseases may also be inferred from host cell response and clinicopathological changes associated with the disease particularly in endemic areas. Various studies have also reported various changes in the biochemical and cellular constituents of blood imposed by haemoparasites [21,22]. Cytological diagnosis has been in the forefront of diagnostic techniques because of its availability as it is rapid, safe, cost-effective [23], easy to carry out and non-invasive. Cytopathology has been used in the diagnosis of many infectious and non-infectious diseases most especially in the detection and identification of common microorganisms in various cytologic specimens [24]. Given that literatures on cytopathological changes associated with these endemic haemoparasitic infections in small ruminants are relatively scanty, this study sought to reinvestigate haemoparasitic infections in small ruminants using PCR and sequencing, evaluate the haematological parameters and to record the morphological changes in blood cells associated with those parasites for an update in the hemoparasite types circulating in the studied area and, to understand their haemocytological changes for effective control and prevention.
Materials and methods
Animals, blood and tissue sample collection
Blood and tissue (liver, spleen and mesenteric lymph node) samples were collected randomly within 6 months from a total of 173 goats, 137 (79.2%) females and 36 (20.8%) males of different ages in ‘Bodija’ Abattoir and ‘Sabo’ slaughter slab (Carrot market) in Ibadan, Oyo State. From each animal, 3mls of blood were collected from the severed jugular vein and dispensed into ethylenediaminetetraacetic acid (EDTA) while a small piece of tissues were harvested. They were transported in cold packs to the laboratory for haematologic, microscopic and Polymerase Chain Reaction (PCR) analyses.
Haematological evaluation
Full blood count, haemoglobin concentration, and total plasma protein were determined from each blood sample using standard haematological techniques [25-27] and as previously described by Happi, et al. [18].
Light microscopic detection of haemoparasites
Giemsa stained thin blood and buffy coat smears were screened for haemoparasites under x1000 magnification (oil immersion) with an Olympus microscope according to the characteristic already described and also reported by Happi, et al. [18].
Erythrocytological evaluation of samples
Giemsa stained thin blood smears were examined under light microscopy at x1000 magnification with immersion oil for red blood cell abnormalities. Quantitation of different erythrocyte abnormalities were recorded over 200 red blood cells. Morphological abnormalities were reported in semi quantitative fashion as previously described [28]. Results of the semi quantitative count of the erythrocyte morphological abnormalities were converted using reference guide [28], to give the descriptive clinical interpretation of the morphological abnormalities or erythrocytological diagnosis associated with the haemoparasitic infections. Percentage animal in each group with erythrocytopathological changes was also estimated.
Cytopathological evaluation of tissue samples
Giemsa stained touch impression smear of the cut tissue surface (liver, spleen and lymph node) harvested were prepared and examined under light microscopy at ×1000 magnification with immersion oil for detection of parasites, inflammatory cells, and cell abnormalities. Quantitation of the different inflammatory cell types per 200 cells was recorded. The percentage of each cell type was used to make cytological diagnoses of various organs. Results obtained were classified based on infection type(s) and the percentage of animals in each group with a type of cytological changes in each group determined.
DNA extraction
DNA extraction and purification from blood obtained from each animal was performed using the QIAamp DNA blood mini kits (QIAGEN Inc. 27220 Turnberry, Lane, Valencia, USA) following the manufacturer’s protocol. DNA templates were extracted from 126 randomly selected samples.
Polymerase chain reaction for detection of haemoparasites
Using a modified protocol [29], the DNA templates were first amplified in 2 series of PCR reactions using 2 sets of selected primer pairs [viz: RLB-F2 (5ꞌ-GAC ACA GGG AGG TAG TGA CAA G-3ꞌ) and RLB-R2 (5ꞌ-CTA AGA ATT TCA CCT CTG ACA GT-3ꞌ)] with an amplicon size of approximately 400bp of the 18S rRNA gene spanning V4 hypervariable region [30], to detect Babesia/Theileria spp, and 16S8FE (5ꞌ- GGA ATT CAG AGT TGG ATC MTG GYT CAG -3ꞌ) and B-GA1B (5ꞌ- CGG GAT CCC GAG TTT GCC GGG ACT TYT TCT -3ꞌ) with an amplicon size of approximately 500bp of the 16S rRNA gene spanning the hypervariable V1 region of the genera to detect Trypanosoma brucei / Ehrlichia [31]. The PCR was done using the touch down program described by Schouls, et al. [31] and modified by Berggoetz, et al. [32], to minimize non-specific amplification. In addition, PCR detection of To-GoTM (Hemoplasma) was performed on 91 randomly selected DNA samples from the 173 goats by a protocol described by Nishizawa, et al. [33], using the primer set HEMOF (5’-ATATTCCTACGGGAAGCAGC-3’) and HEMOR (5’-ACCGCAGCTGCTGGCACATA-3’) [34] with the PCR condition previously described [19] to amplify a 175-195 bp fragment of the 16S rRNA gene of To-GoTM spp.
Briefly, each PCR reaction was performed using the PuReTaq Ready-To-GoTM PCR Beads from GE Healthcare containing approximately 2.5 units PuReTaq polymerase, 200 μM deoxynucleotide triphosphate, Bovine Serum Albumin (BSA), stabilizers and reaction buffer (10 mM Tris-HCl pH 9.0, 50 mM KCl, 1.5 mM MgCl2) as previously described by Happi, et al. [19]. Two positive samples for Hemoplasma, Trypanosoma brucei/ ehrlichia and, Babesia /theileria species each, obtained from the cow [19] were used as positive controls in all PCR reactions while double distilled water was used as negative control. After amplification, 5μl of each amplicon was resolved by electrophoresis on a 1% agarose gel and visualized under ultraviolet trans-illuminator light at 325nm wavelength.
DNA sequencing and phylogenetic analysis for haemoparasite identification
Randomly selected PCR amplicons from each genus (2 from Trypanosoma brucei/ehrlichia, 2 from Babesia/theileria and 10 from To-GoTM) were purified using a Qiaquick PCR purification kit (QIAGEN) and sent for sequencing by Eton Bioscience, Inc, (56 Roland Street, Suite 306 Floor 3R Boston, MA 02129, USA). Each PCR amplicon was sequenced in both forward and reverse directions. The chromatograms of the sequences were viewed using Geneious (v2019.0.4) software (www.geneious.com) [35] and manual base calling was carried out for regions of ambiguities. Following the manual base call, the consensus nucleotide sequences were subjected to Basic Local Alignment Search Tool (BLAST) analysis to identify the organisms and their accession numbers were subsequently submitted to GenBank.
Following BLASTn analysis, the sequence identified as To-GoTM spp. by BLAST analysis was aligned with sixteen (16) established mycoplasma species while the sequence identified as Pseudomonas sp. was aligned with sixteen (16) established pseudomonas species using MAFFT v7.388 [36], with further adjustment made manually as necessary in Geneious [35]. Two neighbor-joining trees [37] were generated also in Geneious from a distance matrix corrected for nucleotide substitutions by the Tamura-Nei model [38]. The trees were viewed and manually edited using FigTree (http://tree.bio.ed.ac.uk/software/figtree/) [39].
Data analysis
Data were double entered serially using codes assigned to individual animal and were analyzed using GraphPad Prism (version 5) and statistical program SPSS for Windows (version 24.0). Categorical variables were compared by calculating χ2 using Yates’ correction, Fisher’s exact or Mantel Haenszel tests. Normally distributed, continuous variables were compared by Student’s t test and analysis of variance (ANOVA). Non-normally distributed, continuous variables were compared by Mann Whitney U test or Kruskal Wallis test. Degree of association between two variables that are continuous and normally distributed and those that are discrete and not normally distributed were evaluated by Pearson’s correlation coefficient and Spearman’s correlation coefficient, respectively. All tests were two-tailed and statistical significance was set at P <0.05.
Results
Haemoparasite detection in goats by light microscopy
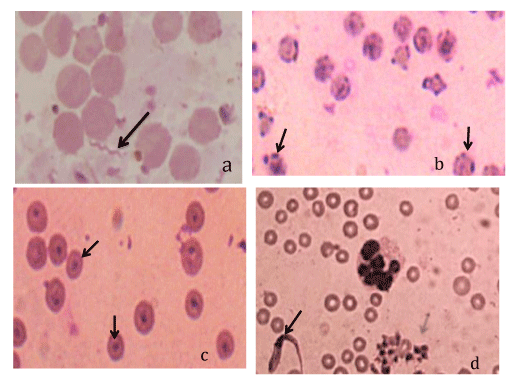
Overall, using the light microscopy (LM), five different haemoparasites were identified in 25 of 173 (14.5%) goats [Borrelia spp (n=1) (Figure 1a), Hemoplasma spp (n=7) (Figure 1b), Trypanosoma brucei spp (n=18) (Figure 1c), Trypanosoma brucei (n=3) (Figure 1d), and Babesia spp (n=2) (image not shown)]. Mono-infections were identified in 20 goats [Trypanosoma brucei spp (n = 14), Hemoplasma spp (n=2), Trypanosoma brucei (n=2), Babesia spp (n=1)] while multiple infections of four different combinations of haemoparasites were identified in five small goats [(Babesia spp + Trypanosoma brucei spp + Hemoplasma spp; n=1); (Borrelia spp + Hemoplasma spp; n=1); (Trypanosoma brucei spp + Hemoplasma spp; n=2); (T. brucei + Trypanosoma brucei spp + Hemoplasma spp; n=1)].
Haemoparasite detection by polymerase chain reaction in goats
Overall, of the 126 samples PCR analyzed for Babesia/Theileria (B/T) and Trypanosoma brucei/Ehrlichia (A/E), amplification was successful in 60 (47.6%). A total of 43/126 (34.1%) and 29/126 (23%) samples were positive for B/T and A/E, respectively. Co-infection of B/T+A/E was observed in 12 of 126 animals (9.5%). Also, PCR targeting To-GoTM spp was positive in a total of 47/91 (51.6%) goats. In the To-GoTM spp positive samples, 16/ 47 (34.0%) were co-infected with A/E or B/T or both and 31 animals had single infection with Hemoplasma spp (H. spp). Double infection of H. spp + A/E and H. spp + B/T were identified in 3 and 10 goats, respectively. Three goats were infected with the three organisms namely: A/E + H. spp + B/T, Bo. spp + H. spp + B/T and T. spp + H. spp + A/E.
Comparison of haemoparasites detected by LM and PCR
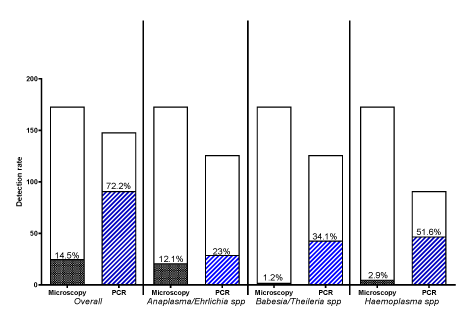
Overall, the detection rate by PCR was significantly higher compared with the LM [91 of 148 (61.5%) versus 25 of 173; (14.5%) samples, respectively; P < 0.0001]. The detection rate by PCR was significantly higher for all the parasite types (P = 0.0008). However, level of difference in detection rate varied across haemoparasites (Figure 2). There was approximately 50% misdiagnosis (under-diagnosis) of Hemoplasma spp by LM (Figure 2) while 82.7% (24/29) and 100% (43/43) were false negative diagnoses for A/E and B/T, respectively. In addition, of the 97 PCR-confirmed negative samples for A/E, 10 were positive by LM (10.3% false positivity recorded for A/E by LM). Also, there was 1.2% (1/83) and 2.3% (1/44) false positivity recorded for B/T and H. spp, respectively.
Haemoparasite detection by both LM and PCR from goats
Based on the higher sensitivity of PCR technique, PCR results were given preference over LM in case of conflicting results in order to obtain the overall infectivity level in the sampled goat by both PCR and LM detection. Haemoparasite identified by both methods were Babesia/Theileria spp, Trypanosoma brucei/Ehrlichia spp, Hemoplasma spp, Trypanosoma spp, and Borellia spp. A total of 150 goats were tested by both methods. Estimated overall infection rate was 62.7% with at least one haemoparasite. In haemoparasite-infected goats, infection with Hemoplasma spp (51.6%) was the most occurring infection type followed by Babesia/Theileria spp (34.1%). The proportion of single infection with the PCR detected haemoparasites was higher than co-infections (Table 1). Single infection with Hemoplasma spp, Babesia/Theileria spp and Trypanosoma brucei/Ehrlichia spp were 34.1%, 22.0% and 14.3%, respectively (Table 1)..
Haemoparasite infectivity based on gender, age and breed of goats
There was no significant difference (P>0.05) between the age, gender and breed of haemoparasite-infected and non-infected goats (Table 1). In addition, these factors were similar in goats infected with the various haemoparasites.
Haematological findings in haemoparasite-infected goats in Ibadan
When comparing the haematological parameters of PCR-confirmed heamoparasite positive and negative goats, only the TWBC and lymphocyte counts, showed significant differences. The mean TWBC count and mean lymphocyte counts were significantly higher in PCR-confirmed haemoparasite-infected goats compared to the haemoparasite negative ones (6869 (95%CI 6333 – 7405) versus 5932 (95%CI 5353-6511), respectively; P = 0.02) and 3627 (95%CI 3238 – 4016) versus 3019 (95%CI 2632 – 3408), respectively; P = 0.04).
Blood cell abnormalities
Overall, blood cell abnormalities were observed in 38 goats (25.7%). Of these, multiple cell abnormalities were observed in 17 goats (44.7%) while 21 (56%) had single abnormalities. The morphologic abnormalities recorded included: Poikilocytosis (n = 3), Keratocytosis (n = 2), hypochromasia (n = 2), mega platelets (n = 3), anisocytosis (n = 8), ovalocytosis (n = 6), ecchynocytes (n = 19) and dacrocytes (n = 9). Hypersegmented neutrophils were found in blood smears of goats with mixed haemoparasitic infections (B/T+A/E and T+A/E+H. spp). Apoptotic neutrophils were observed in 50% of H. spp and 5.9% of A/E infected-goats. In a pool analysis of all abnormalities, proportion of animals with blood cell abnormalities was similar in infected and non-infected goats (19 of 91 versus 16 of 57 animals, respectively; P = 0.49). When analysed separately, distribution of various blood cell abnormalities were also similar in infected and non-infected goats. However, the degree and number blood cell abnormalities varied in haemoparasite-infected and haemoparasite negative goats. Thus, there was no correlation between blood cell changes and the haemoparasite infection type and breed.
Cytopathology of lymph nodes, spleens and livers associated with haemoparasitic infections in goats
The cytopathological changes observed in the spleens, lymph nodes and livers of goats infected with both single and multiple haemoparasites were not significantly different, nor specific to any infection type, they varied from normal cytology to necrosis, hyperplasia, degeneration, inflammation and lymphomatous reaction. In addition, there was no significant difference in the cytopathological changes recorded in haemoparasite positive and haemoparasite negative animals.
In the spleen, the most consistent cytopathological findings were acute to subacute splenitis in most single and mixed haemoparasitic infections except in T. brucei-infected goats which showed only splenic hyperplasia with erythrophagocytosis and haemosiderosis (100%). However, in B/T positive goats, 26% showed splenitis, 3.3% lymphomatous reaction and 6.7% of spleens were normal. In the A/E infected group, 35.3% of spleen was hyperplastic while 41.2% were normal. In B/T+A/E positive group, 25.0% showed histiocytic splenitis and 18.8% were normal spleen. Other cytopathological changes recorded in the spleen were Mott cells in T. spp +H. spp +A/E infected animals.
The livers in most infections showed hepatitis while a few showed fatty change (vacuolar hepatocytes) and others were normal.
The lymph nodes of 35.3% haemoparasite infected animals showed lymphoid hyperplasia while 28.3% revealed an acute lymphandenitis, and 33.3% had normal lymph node. Lymphomatous lymph node was recorded in one case of A/E positive goat. In addition, a morula was observed in the lymph node of a goat positive for A/E with a few mitotic lymphocytes and binucleated plasma cells. Other changes in tissue smear were erythrophagocytosis and haemosiderosis in the lymph node of B/T+A/E infected goat. In most aparasitaemic goats, the spleen, lymph node and liver showed normal cytological morphology.
Molecular characterization of haemoparasites
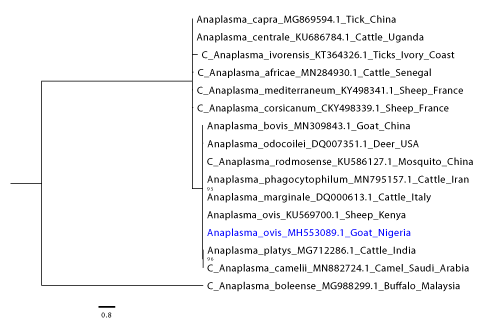
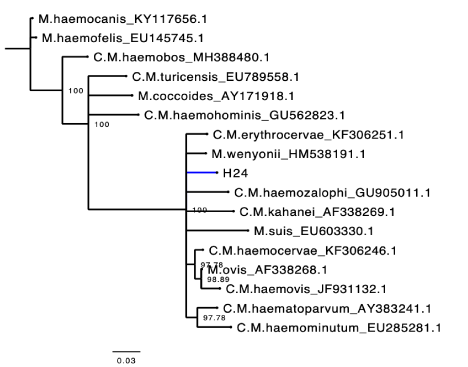
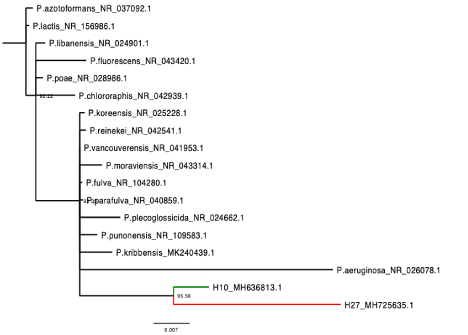
The field isolate of A/E sequenced amplicons (accession number: MH553089.1) fell in the same clade and clustered closely with an Trypanosoma brucei ovis sequence obtained from a sheep in Kenya with whom it also shared a high pairwise identity (100%) (Figure 3). While in our To-GoTM isolates, a sequence shared 100% homology with the published Mycoplasma ovis strain Kamosika3 and Mycoplasma wenyonii (MK484136) from the NCBI database. On the phylogenetic tree, that Mycoplasma wenyonii clustered into the same clade with Mycoplasma wenyonii (HM 538191.1. obtained from a buffalo in China) (Figure 4). Incidentally, two of the Hemoplasma sequence from our study shared high (96 and 98.5 %) homology with Pseudomonas fluorescens (MH725635) (only the one with 98.5% homology was submitted) from the NBCI database (Figure 5). Among the two Babesia /Theileria spp sequenced one showed 99% homology with Theileria velifera from the NCBI database and the other one had low grade and low pairwise percentage insufficient to include in our findings.
Discussion
Haemoparasites are endemic in sheep and goats in tropical and subtropical regions of the world [40]. However, information about their abundance and distribution in Southern Nigeria (Ibadan) is limited. In addition, species differentiations is challenging among some haemoparasites using LM. The little information available on the prevalence has been obtained using LM [41-46]. The hemoparasites commonly reported in small ruminants were Trypanosoma brucei, Babesia, Theileria [41-44], and scanty reports of Eperythrozoon species [41] and Trypanosoma species [43,45]. In this study, LM and the molecular techniques were used to detect the haemoparasites types with the view to give an update in their prevalence and the different species found in Southern Nigeria (Ibadan). The infection rate recorded was lower with LM compared to the PCR. Similar differences have been reported in other studies, which confirm the lower sensitivity of LM. However, more haemoparasite types were detected with the LM than with the PCR method because the primer sets used were for Babesia/Theileria, Trypanosoma brucei/Ehrlichia, and To-GoTM only.
On the whole, the study disclosed high infection rates (62.7%) in the goat population by both LM and PCR. The higher prevalence in the sampled goats could be attributed to the abundance of the vectors involved in the transmission of these parasites due to suitable environmental conditions appropriate for their survival [5] and the higher sensitivity (PCR) method used [47-50]. The overall prevalence rate recorded during this study in small ruminants is higher than anyone ever reported in Nigeria [41-46,51-53]. In addition the 7 haemoparasite species recorded are more numerous than the previously reported ones [41-46,51-55].
The numerous haemoparasite types observed are as a result of vectors common to most types of haemoparasitism recorded (except T. brucei.) and the environmental, and ecologic factors, which favor presence of tick population in the study area, as well as the combine methods of detection (LM and PCR).
These results revealed that To-GoTM spp is the most prevalent blood parasite (51.6%). This is also the first time To-GoTM is reported as the most abundant haemoparasites hosted by small domestic ruminants in Nigeria and Africa as a whole.
Trypanosoma brucei ovis, T. brucei, and Mycoplasma ovis (formerly Eperithrozoon ovis) have been the haemoparasites most reported in small ruminants worldwide [59,62], including Nigeria [41,51], causing significant economic loss among farmers. A report of Mycoplasma wenyonii (common To-GoTM of bovine) infection with clinical manifestations in sheep has been published in Turkey [63]. However, in Nigeria it has been reported only in cattle [19]. Theileria velifera also known to infect bovine and buffalos in many countries [64-66] has also recently been reported from cattle in Ibadan, Nigeria [19]. This cross species infections may be because most ruminant farmers in Nigeria keep sheep, goat and cattle together for more profit. Therefore their common vectors (ticks) may move from one host species to the other and transmit the parasites indiscriminately among animals kept together. Thus there is need for epidemiological survey of T. velifera as well as Mycoplasma wenyonii among small rumimants together with their pathogeniticity and zoonotic implications.
The report of Borrelia theileri infection in Nigeria is very rare and has been reported in cattle in 1927 and sheep in 1967 [67]. It has recently been reported in sheep in Ghana [2] while Borrelia burgdorferi the cause of a zoonosis called “Lyme’s Disease” is commonly found in animals such as cow, and has been reported in sheep, horses, rats and birds in USA (Winscosin), China, and Europe [68-70]. Borrelia burgdorferi infection is a common finding in Wisconsin, while Borrelia theileri has been identified in cattle, sheep and horses in South Africa. In Ghana, the Borrelia specie found was not characterized. However, due to the movement of many animals from South Africa to Nigeria, the Borrelia spp herewith recorded could be similar to the South African reported parasite. In addition, given the morphology of the Borrelia observed during this study, Borrelia theileri is suggested to be the specie found, although was observed in goat. Thus, there is need for further epidemiological investigation as well as study of the pathogenicity, their economic and zoonotic implication in small animals farming and farmers.
There was no significant difference in most haematological parameters in haemoparasite positive and negative small ruminants except for the mild increase in the TWBC .the increase was due to increase in neutrophils and lymphocyte counts particularly in the A/E infected group. These suggest that A/E infection stimulates an inflammatory and or immunologic response in the hosts
Blood cell abnormalities were observed in the haemoparasite-infected and non-infected goats. They varied and showed no peculiar morphological changes specific to any infection type. Poikilocytosis and anisocytosis were blood cell abnormalities observed in most of the haemoparasite positive goats. They are usually observed in regenerative anemia. Given that haemoparasitic infections cause intra or extravascular haemolysis, it suggests there is a low grade anaemia despite the absence of statistic difference between both groups. In addition, the infection may be benign or the haemoparasite negative ones are also harboring other anemia causing organisms (such as hook worms) or they are relatively tolerant/carriers to the haemoparasites and may succumb when they have concurrent infection or the hosts are immunosuppressed.
A few Apoptotic neutrophils were observed in the blood smears of H. spp and of A/E infected goats. White blood cell apoptosis is a rare finding in haemoparasite-infected hosts, there are a few reports of blood cell apoptosis in T. brucei [71-73], Plasmodium spp [74,75], T. gondii [76,77], T. cruzi [78-80] and E. histolytica [81] infected hosts, where they are described to be involved in the pathogenesis of the disease and may contribute to host susceptibility to infection. From this result, future studies aimed at investigating haemoparasite-induced apoptosis of neutrophils in haemoplasma and A/E infection and their implication in the pathogenesis may be envisaged.
The cytopathological changes observed in the tissues of goats infected with haemoparasitic infections were not specific to an infection type/group of infections, they varied from normal cytology to necrosis, hyperplasia, degeneration, inflammation and lymphomatous reaction. In this study, 100% of the population of Trypanosoma brucei and To-GoTM infected goats had hyperplastic lymph nodes and this finding is in agreement with those of [82,83] who reported hyperplasia in the lymph nodes of humans infected with Trypanosoma brucei. The liver tissues of most of these animals, showed more of chronic active hepatitis and fatty degenerative changes especially in those with single haemoparasitic infection (B/T and A/E). This is similar with the reports of [47,84,85], who associated anaplasmosis with degeneration and necrosis of the hepatocytes, secondary to anaemic hypoxia. However, subacute hepatitis was also observed in the liver of T. brucei infected goats in this study and this is in contradiction with the findings of [86] who reported necrosis of hepatocytes only.
Trypanosoma infection in this study was associated with hyperplasia of the spleen. This is in agreement with the findings of [87-90], but different from the findings of Uche and John [88], who reported degenerative changes in the spleen of trypanosoma infected animals. Hyperplasia is an indication of an immunological reaction and increased production of immunoglobulin. It has been established that Babesia and theileria are associated with hyperplasia of the spleen and lymph node in animals [91]. This is similar with the findings from this study, although some of the goats infected with Babesia/Theileria also showed splenitis. Other changes in tissue smear such as erythrophagocytosis by macrophage and haemosiderosis in the lymph node of B/T+A/E have been reported by Anosa and Kaneko [89], in deer mice infected with trypanosome spp, [90] and in the spleen of T. brucei infected Wistar rats and goats [72,73]. The Binucleated and mitotic lymphocytes with numerous plasma cells recorded in the lymph nodes of goats infected with A/E and B/T have also been reported in the lymph nodes of T. brucei infected Wistar rats and goats [72,73]. This implies an active or responsive lymph node to parasitic or non-parasitic stimulations.
A sequence from our study shared highest homology (100%) with Mycoplasma ovis and Mycoplasma wenyonii from the NCBI database and on the phylogenetic tree it fell in the Wenyonii (or Haemominutum) cluster. Previous studies have shown that haemoplasmas are divided into two phylogenetic clusters, Wenyonii (Haemominutum) and Hemofelis [92,93]. Incidentally, a sequence from our study shared high homology (98.5%) with several species of Pseudomonas particularly Pseudomonas fluorescens from the NCBI database. In a recent study, the first report of P. fluorescens in cattle in Nigeria and Africa was made [19]. This might imply that P. fluorescens is more prevalent in Nigerian animals than aforethought and further investigative studies need to be carried out to determine their prevalence and pathogenicity in infected hosts. Interestingly also, on the phylogenetic tree, the sequence from this study shared a most recent common ancestor with the sequence from our previous study and a 99% pairwise identity implying the organism might have found its way into the other animal species from a common source which maybe while grazing on plants or drinking water. This is because many species of pseudomonas are commonly found in soil, water or plant surfaces and have been known to either cause infection in plants/plant products or been used as biological control of plant pathogens [94-102].
Conclusion
Haemoparasitic infections are common in small ruminants in Nigeria. The striking disparity in the LM and PCR methods of detection also highlights the need to consider at least light microscopy and PCR detection methods for haemoparasite diagnosis as they may provide more accurate results. The first documentation of To-GoTM species as the most prevalent haemoparasite types in infected goats in the studied area is presented. In addition, the demonstration of Mycoplasma wenyonii, Theileria velifera and Pseudomonas fluorescens infections in the small ruminants here presented is unusual and suggest species cross over. Herein, the results also show that the haemocytopathology and cytopathology of spleen, liver and lymph node during haemoparasitic infection is substantially limited in respect to specific diagnosis hence aetiologic diagnosis cannot be made from blood cell morphologic alteration or tissue response. Studies gear towards the investigation of their pathogenicity in the novel host, their origin, diversity and factor of transmission are envisaged for effective prevention and control of these potentially zoonotic organisms.
- Akerejola OO, Schillhorn van Veen TW, Njoku CO (1979) Ovine and caprine diseases in Nigeria: a review of economic losses. Bull Animal Health Prod Afri 27: 65-70. Link: https://bit.ly/2VA9p53
- Bell-Sakyi L, Koney EBM, Dogbey O, Walker AR (2004) Incidence and prevalence of tick-borne haemoparasites in domestic ruminants in Ghana. Vet Parasitol 124: 25-42. Link: https://bit.ly/3dQWIsW
- Okaiyeto SO, Tekdek LB, Sackey AKB, Ajanusi OJ (2008) Prevalence of haemo and gastrointestinal parasites in sheep and goats kept by the Normadic Fulanis in some Northern states of Nigeria. Res J Anim Sci 2: 31-35. Link: https://bit.ly/2YQaJTn
- Takeet MI, Akande FA, Abakpa SAV (2009) Haemoprotozoan parasites of sheep in Abeokuta, Nigeria. Nig J Parasitol30: 142-146.
- Akande FA, Takeet MI, Makanju OA (2010) Haemoparasites of cattle in Abeokuta, South West Nigeria. Sci World J 5: 19-21. Link: https://bit.ly/3dR6B9O
- Simuunza MC (2009) Differential diagnosis of tick-borne diseases and population genetic analysis of Babesia bovis and Babesia bigemina. University of Glasgow. Link: https://bit.ly/38jLI6b
- Ameen KAH, Abdullah BA, Abdul-Razaq RA (2012) Seroprevalence of Babesia bigemina and Trypanosoma brucei marginale in domestic animals in Erbil, Iraq. Iraqi J Vet Sci 26: 109-114. Link: https://bit.ly/2Zoz3uA
- Domingos A, Antunes S, Borges L, Rosário VE (2013) Approaches towards tick and tick-borne diseases control. Rev Soc Bras Med Trop 46: 3. Link: https://bit.ly/2BqaDsA
- Hooshmand-Rad P, Hawa NJ (1973) Malignant theileriosis of sheep and goats. Trop Anim Health Prod 5: 97–102. Link: https://bit.ly/2ZpJD4B
- Perry BD, Randolph TF (1999) Improving the assessment of the economic impact of parasitic diseases and of their control in production animals. Vet Parasitol 84: 145-168. Link: https://bit.ly/2YTfETL
- Oluwafemi TU, Anosa VO (2000) Haematological studies on domestic animals in Nigeria, clinico–haematological features of bovine trypanosomiasis, theileriosis, anaplasmosis, eperythrozoonosis and helminthiasis. Ziblatt Vet Med 2: 789 -797. Link: https://bit.ly/3ihQd5L
- Hanson J, Perry B (1994) The Epidemiology, Diagnosis and Control of Helminth Parasites of Ruminants. A Hand Book, Food and Agriculture Organization of the United Nations, Rome, Italy. 72-89. Link: https://bit.ly/2BiznDm
- Luckins AG (1992) Trypanosomosis in small ruminants–a major constraint to livestock production? Brit Vet J 148: 471– 472. Link: https://bit.ly/31yIF8F
- Mahmoud MM, El-malik KH (1977) Trypanosomiasis: goats as a possible reservoir of Trypanosoma congolense in the republic of the Sudan. Trop Anim Health and Prod 9: 167-170. Link: https://bit.ly/2NKE8rF
- Katunguka-Rwakishaya E (1996) The prevalence of trypanosomosis in small ruminants and pigs in a sleeping sickness endemic area. Rev Elev Med Vet Pays Trop 49: 56-58. Link: https://bit.ly/2BrQT83
- Omotainse SO, Edeghere H, Omoogum GA, Elhassan EO, Thompson G, et al. (2000) The prevalence of animal trypanosomosis in Konshisha Local Government Area of Benue State, Nigeria. Israel J Vet Med 55: 142-144. Link: https://bit.ly/2NLkQm6
- Tambuwal FM, Agaie BM, Bangana A (2002) Haematological and biochemical values of apparently healthy red Sokoto goats. Proceeding of 27th Annual Conference, Nigerian Society of Animal production (NSAP) Nigeria. 50-53.
- Happi NA, Toepp AJ, Ugwu CA, Petersen CA, Sykes JE (2018) Detection and identification of blood-borne infections in dogs in Nigeria using light microscopy and the polymerase chain reaction. Vet Parasitol Reg Stud Rep 11: 55–60. Link: https://bit.ly/2BVoJCe
- Happi AN, Osifade O, Oluniyi PE, Ogunro BN (2019) Comparison of Light Microscopy and Polymerase Chain Reaction for the Detection of Haemoparasites in Cattle in Nigeria. Acta Parasitol 65: 44-56. Link: https://bit.ly/2NN78is
- Carelli G, Decaro N, Lorusso A, Elia G, Lorusso E, et al. (2007) Detection and quantification of Trypanosoma brucei marginale DNA in blood samples of cattle by realtime PCR. Vet Microbiol 124: 107-114. Link: https://bit.ly/2NHr7z8
- Igbokwe IO, Mohammed A (1992) Some plasma biochemical changes in experimental Trypanosoma brucei infection in Sokoto red goats. Revue Elev Med Vet Pays Trop 45: 287-290. Link: https://bit.ly/38mccDZ
- Taiwo VO, Olaniyi MO, Ogunsanmi AO (2003) Comparative plasma biochemical changes and susceptibility of erythrocytes to invitro peroxidation during experimental Trypanosoma congolense and T. brucei infections in sheep. Isr J Vet Med 58: 435-443.
- Bottles K, McPhaul LW, Volberding P (1988) Fine-needle aspiration biopsy of patients with acquired immunodeficiency syndrome (AIDS): experience in an outpatient clinic. Ann Intern Med 108: 42-45. Link: https://bit.ly/3ilPHUg
- Celeste NP (1998) Diagnosis of infectious diseases: a cytopathologist’s perspective. Clin Microbiol Rev 11: 2. Link: https://bit.ly/31xaOgm
- Schalm OW, Jain NC, Carrol EJ (1975) Veterinary haematology. 3rd Edition. Lea and Febiger, Philadephia.
- Coles EH (1980) Veterinary clinical pathology, 3rd Edition. W.B. Sanders Co. Philadelphia.
- Jain NC (1986) Schalm ́s Veterinary Hematology. Lea and Febiger, Philadelphia 221. Link: https://bit.ly/2Zu3pf5
- Weiss DJ (1984) Uniform evaluation and semiquantitative reporting of hematologic data in veterinary laboratories. Vet Clin Pathol 13: 27-31. Link: https://bit.ly/3dWGjTX
- Bekker CPJ, Bell-Sakyi L, Paxton EA, Martinez D, Bensaid A, et al. (2002) Transcriptional analysis of the major antigenic protein 1 multigene family of Cowdria ruminantium. Gene 285: 193-201. Link: https://bit.ly/2YRj9Km
- Georges K, Loria GR, Riili S, Greco A, Caracappa S, et al. (2001) Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet Parasitol 99: 273–286. Link: https://bit.ly/31y726t
- Schouls LM, De Pol IV, Rijpkema SGT, Schot C (1999) Detection and Identification of Ehrlichia, Borrelia burgdorferi Sensu Lato, and Bartonella Species in Dutch Ixodes ricinus Ticks. J clin Microbiol 37: 2215-2222. Link: https://bit.ly/31ziQ8I
- Berggoetz M, Schmid M, Ston D, Wyss V, Chevillon C, et al. (2014) Tick-borne pathogens in the blood of wild and domestic ungulates in South Africa: interplay of game and livestock. Ticks Tick Borne Dis 5: 166-175. Link: https://bit.ly/2BvsIFE
- Nishizawa I, Sato M, Fujihara M, Sato S, Harasawa R (2010) Differential detection of To-GoTM species in cattle by melting curve analysis of PCR products. J Vet Med Sci 72: 77-79. Link: https://bit.ly/2VCAZyx
- Fujihara Y, Sasaoka F, Suzuki J, Watanabe Y, Fujihara M, et al. (2011) Prevalence of Hemoplasma infection among cattle in the western part of Japan. J Vet Med Sci 73: 1653-1655. Link: https://bit.ly/2BrV3wK
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, et al. (2012) Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. J Bioinform 28: 1647-1649. Link: https://bit.ly/2VD4Ab0
- Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30: 3059-3066. Link: https://bit.ly/3ij66sx
- Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4: 406-425. Link: https://bit.ly/3gh4zBs
- Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10: 512-526. Link: https://bit.ly/2YOFU1l
- FigTree (http://tree.bio.ed.ac.uk/software/figtree/).
- Ros-Garcia A, Barandika JF, Garcia-Perez AL, Juste RA, Hurtado A (2013) Assessmentof exposure to piroplasms in sheep grazing in communal mountainpastures by using a multiplex DNA bead-based suspension array. Parasit Vectors 6: 277. Link: https://bit.ly/2BYF497
- Adejinmi JO, Sadiq NA, Fashanu SO, Lasisi OT, Ekundayo S (2004) Studies on the blood parasites of sheep in Ibadan, Nigeria. Afr J Biomed Res 7: 41-43. Link: https://bit.ly/2VwNAmQ
- Adamu BS, Balarabe LM (2012) Prevalence of haemoparasites of sheep and goats slaughtered in Bauchi State Abattoir. Int J Appl Biol Res 4: 128-133.
- Josiah GJ, Omalu ICJ, Makun HJ, Chiezey NP, Abah OOI (2015) Haemonchosis and haemoparasites of small ruminants reared in north western, Nigeria. Anim Res Int 12: 2284-2291.
- Anyanwu NCJ, Iheanacho CN, Adogo LY (2016) Parasitological Screening of Haemo-Parasites of Small Ruminants in Karu Local Government Area of Nasarawa State, Nigeria. Br Microbiol Res J 11: 1-8. Link: https://bit.ly/38teKR3
- Bello AM, Lawal JR, Dauda J, Wakil Y, Mshellia ES, et al. (2017) Prevalence of haemoparasites in Balami sheep from Maiduguri, Northeastern Nigeria. Direct Res J Vet Med Anim Sci 2: 28-35. Link: https://bit.ly/3dPLgh8
- David OFS, Goria KP, Abraham DGA (2018) Haemoparasite fauna of domestic animals in plateau state, north central Nigeria. BAJOPAS 11: 156-161. Link: https://bit.ly/31Amz60
- Radostis OM, Gay CC, Hinchcliff KW, Constable PD (2007) Veterinary Medicine: A Textbook of the Diseases of Cattle, Horse, Sheep, Pigs, and Goats. 10th Ed. Saunders Elsevier, Edinburgh, UK.
- Aktas M, Altay K, Dumanli N (2005) Survey of theileria parasites of sheep in eastern Turkey using polymerase chain reaction. Small Rumin Res 60: 289-293. Link: https://bit.ly/3eKYAoh
- Altay K, Dumanli N, Holman PJ, Aktas M (2005) Detection of Theileria ovis in naturally infected sheep by nested PCR. Vet Parasitol 127: 99-104. Link: https://bit.ly/3ijrcqT
- Nagore D, Garcia-Sanmartin J, Garcia-Perez AL, Juste RA, Hurtado A (2004) Identification, genetic diversity and prevalence of Theileria and Babesia species in a sheep population from Northern Spain. Int J Parasitol 34: 1059-1067. Link: https://bit.ly/3iorpcy
- Ademola IO, Onyiche TE (2013) Haemoparasites and haematological parameters of slaughtered ruminants and pigs at Bodija abattoir, Ibadan, Nigeria. Afr J Biomed Res 16: 101-105. Link: https://bit.ly/2NIdUpO
- Biu AA, Ibrahim A, Kumshe HA, Ahmed TB (2012) Prevalence of Babesia ovis in Maiduguri Metropolis, Nigeria. J Sci Multidiscip Res 4.
- Opara MN, Nwokedi CC (2011) Occurrence of Haemoparasites among Small Ruminants Reared Under Traditional Husbandry System in Owerri, Southeast Nigerian. AJOL 59: 4. Link: https://bit.ly/2YN6bwY
- Mohammed G, Idoko IS (2012) Haemoparasites & haematological evaluations in Sokoto red goats. Niger Vet J 33: 407-415. Link: https://bit.ly/2Bk4AGh
- Lorusso V, Picozzi K, de Bronsvoort BM, Majekodunmi A, Dongkum C, et al. (2013) Ixodid ticks of traditionally managed cattle in central Nigeria: where Rhipicephalus (Boophilus) microplus does not dare (yet?). Parasite Vector 6: 171. Link: https://bit.ly/2ZFYEj1
- Iqbal F, Fatima M, Shahnawaz S, Naeem M, Shaikh RS, et al. (2011) A study on the determination of risk factors associated with babesiosis and prevalence of Babesia sp., by PCR amplification, in small ruminants from Southern Punjab (Pakistan). Parasite 18: 229-234. Link:
- Samaila AB, Musa BL (2012) Prevalence of haemoparasites of sheep and goats slaughtered in Bauchi Abattoir. IJABR 4: 128-133.
- Opara MN, Ike KA, Okoli IC (2010) Haematological parameters and blood chemistry of apparently healthy West African Dwarf (Wad) goats in Owerri, South Eastern Nigeria. N Y Sci J 3: 8. Link: https://bit.ly/3idqxam
- Bilgic HB, Bakırcı S, Kose O, Unlu AH, Hacılarlıoglu S, et al. (2017) Prevalence of tick-borne haemoparasites in small ruminants in Turkey and diagnostic sensitivity of single-PCR and RLB. Parasite Vector 10: 211. Link: https://bit.ly/31wIpXW
- Naz S, Maqbool A, Ahmed S, Ashraf K, Saeed K, et al. (2012) Prevalence of theileriosis in small ruminants in Lahore- Pakistan. J Vet Anim Sci 2: 16-20. Link: https://bit.ly/2NNxr8d
- Friedhoff KT (1997) Tick-borne diseases of sheep and goats caused by Babesia, Theileria or Trypanosoma brucei spp. Parassitologia 39: 99–109. Link: https://bit.ly/2ZqtMmm
- Stuen S (2017) Haemoparasitism of Goats and Sheep. In: Sustainable Goat Production in Adverse Environments I 293–315. Link: https://bit.ly/38kOGHv
- Aktas M, Ozubek S (2017) A molecular survey of small ruminant hemotropic mycoplasmosis in Turkey, including first laboratory confirmed clinical cases caused by Mycoplasma ovis. Vet microbiol 208: 217-222. Link: https://bit.ly/2NKJO4X
- Eygelaar D, Jori F, Mokopasetso M, Sibeko KP, Collins NE, et al. (2015) Tick-borne haemoparasites in African buffalo (Syncerus caffer) from two wildlife areas in Northern Botswana. Parasite Vector 8: 26. Link: https://bit.ly/31AA99p
- Stoltsz WH (1996) Theileriosis in the African Buffalo. In: Proceedings of a Symposium on the African Buffalo as a Game Ranch Animal, Wildlife Group of the South African Veterinary Association. South Africa: Onderstepoort 126-130.
- Norval RAI, Perry BD, Young AS (1992) The Epidemiology of Theileriosis in Africa. London: Academic Press Link: https://bit.ly/2Zv2TxG
- Leeflang P, Ilemobade AA (1977) Tick-borne diseases of domestic animals in northern Nigeria. Trop Anim Health Prod 9: 211-218. Link: https://bit.ly/2BYV5w8
- Stoebel K, Schoenberg A, Streich WJ (2003) The seroepidemiology of Lyme borreliosis in zoo animals in Germany. Epidemiol Infect, 131: 975-983. Link: https://bit.ly/3gi2BAP
- Parker JL, White KK (1992) Lyme Borreliosis in cattle and horses: a review of the literature. Cornell Vet 82: 253-274. Link: https://bit.ly/3iiYAOu
- Rizzoli A, Hauffe H, Carpi G, Vourc HG, Neteler M, et al. (2011) Lyme borreliosis in Europe. Euro Surveill 16: 19906. Link: https://bit.ly/2CV0NPS
- Radwanska M, Guirnalda P, De Trez C, Ryffel B, Black S, et al. (2008) Trypanosomiasis-Induced B Cell Apoptosis Results in Loss of Protective Anti-Parasite Antibody Responses and Abolishment of Vaccine-Induced Memory Responses. PLoS Pathog 4: 1000078. Link: https://bit.ly/2YPAHXd
- Happi AN, Milner DA, Antia RE (2012) Blood and tissue leukocyte apoptosis in T. brucei infected rats. J Neuroinfect Dis 3: 10. Link: https://bit.ly/3dPjmSr
- Happi NA, Eden AR, Milner D (2016) Trypanosoma brucei-Induced apoptosis of leucocytes as a factor of trypanosusceptibility in infected goats. Trop Biomed 33: 209-225. Link: https://bit.ly/2VB2VTu
- Toure-Baldo A, Sarthou JL, Roussilhon C (1995) Acute Plasmodium falciparum infection is associated with increased percentage of apoptosis of apoptotic cells. Immunol Lett 46: 59-62. Link: https://bit.ly/2VzmHyP
- Helmby H, Jonsson G, Troye-Blomberg M (2000) Cellular Changes and Apoptosis in the Spleens and Peripheral Blood of Mice Infected with Blood-Stage Plasmodium chabaudi chabaudi AS. Infect Immun 68: 1485-1490. Link: https://bit.ly/3iizdMP
- Khan IA, Matsuura T, Kasper LH (1996) Activation-mediated CD4+ T cell unresponsi-veness during acute Toxoplasma gondii infection in mice. Int Immunol 8: 887-896. Link: https://bit.ly/3dJ6JZ6
- Liesenfeld O, Kosek JC, Suzuki Y (1997) Gamma interferon induces Fas-dependent apoptosis of Peyer's patch T cells in mice following peroral infection with Toxoplasma gondii. Infect Immun 65: 4682-4689. Link: https://bit.ly/2Zu9ND5
- Martin TM, Pedro OC, Caldeira RA, do Rosário VE, Neves L, et al. (2008) Detection of bovine babesiosis in Mozambique by a novel seminested hot-start PCR method. Vet Parasitol 153: 225-230. Link: https://bit.ly/3eSC9xz
- Zuñiga E, Motran CC, Montes CL, Yagita H, Gruppi A (2002) Trypanosoma cruzi infection selectively renders parasite-specific IgG+ B lymphocytes susceptible to Fas/Fas ligand-mediated fratricide. J Immunol 168: 3965-3973. Link: https://bit.ly/2YPms4w
- Silva EM, Guillermo LV, Ribeiro-Gomes FL, De Meis J, Nunes MP, et al. (2007) Caspase inhibition reduces lymphocyte apoptosis and improves host immune responses to Trypanosoma cruzi infection. Eur J Immunol 37: 738-746. Link: https://bit.ly/3eYx6vG
- Huston CD, Boettner DR, Miller-Sims V, Petri WA (2003) Apoptotic killing and phagocytosis of host cells by the parasite Entamoeba histolytica. Infect Immun 71: 964–972. Link: https://bit.ly/3ikXUrS
- John JL, Macnamara KC, Walker NJ, Winslow GM, Borjesson DL (2009) Infection with Trypanosoma brucei phagocytophilum induces multilineage alterations in the haematopoietic progenitor cells and peripheral blood cells. Infect Immune 77: 4070-4080. Link: https://bit.ly/3dMy6S0
- Stephen JD, Kyoung SC, Jose CG, Nicole SB, Diana GS, et al. (2005) Human granulocytic anaplasmosis and Trypanosoma brucei phagocytophilum. Emerg Infect Dis 11: 1828-1834. Link: https://bit.ly/38iMUH3
- Jubb, Kennedy, Palmer. (1991). Pathology of domestic animals. Link:
- Timoney, Gillespie, Scott. (1988). Microbiology and infectious diseases of domestic animals. Link:
- Audu PA, Esievo KAN, Mohammed G, Ajanusi OJ, Ibrahim NDG (1999) Pathological observations in Trypanosoma evansi infected Yankasa sheep. J Protozool Res 9: 64-70. Link: https://bit.ly/2VEegSL
- Losos GJ, Ikede BO (1972) A review of pathology of diseases in domestic and laboratory animals caused by Trypanosoma congolense, T. brucei, T. rhodensiense and T. gambiense. Vet Pathol: 9: 1-71. Link: https://bit.ly/3ipLChR
- Uche UE, Jones TW (1992) Pathology of experimental T. evansi infection in rabbits. J Comp Pathol 106: 299-309. Link: https://bit.ly/3dThl7A
- Anosa VO, Kaneko JJ (1983) Pathogenesis of Trypanosoma brucei infection in deer mice (Peromyscus maniculatus): hematologic, erythrocyte biochemical, and iron metabolic aspects. Am J Vet Res 44: 639–644. Link: https://bit.ly/2VCKcXv
- Taylor K, Authie E, M.-L. (2004) Pathogenesis of animal trypanosomiasis, in The Trypanosomiases, I. Mauldin, P. H.Holmes, and M. A. Miles, eds., CABI, Oxfordshire, UK.
- Jones TC, Hunt RD, King NW (1997) Veterinary pathology. Sixth edition; Pathogenesis and general findings of trypanosomosis. 585-586.
- Peters IR, Helps CR, McAuliffe L, Neimark H, Lappin MR, et al. (2008) RNase P RNA gene (rnpB) phylogeny of Hemoplasmas and other Mycoplasma species. J Clin Microb 46: 1873-1877. Link: https://bit.ly/3dSIQOA
- Watanabe Y, Fujihara M, Obara H, Matsubara K, Yamauchi K, et al. (2010) Novel Hemoplasma species detected in free-ranging sika deer (Cervus nippon). J Vet Med Sci 72: 1527-1530. Link: https://bit.ly/3gaKtJg
- Kwon SW, Kim JS, Park IC, Yoon SH, Park DH, et al. (2003) Pseudomonas koreensis sp. nov., Pseudomonas umsongensis sp. nov. and Pseudomonas jinjuensis sp. nov., novel species from farm soils in Korea. Int J Syst Evol Microbiol 53: 21-27. Link: https://bit.ly/31DcuoS
- Ganeshan G, Kumar AM (2005) Pseudomonas fluorescens, a potential bacterial antagonist to control plant diseases. J Plant Interact 1: 123-134. Link: https://bit.ly/2NPsZWM
- Chiesa F, Lomonaco S, Nucera D, Garoglio D, Dalmasso A, et al. (2014) Distribution of Pseudomonas species in a dairy plant affected by occasional blue discoloration. Int J Food Saf 3: 1722. Link: https://bit.ly/31Cfy4k
- Cho ST, Chang HH, Egamberdieva D, Kamilova F, Lugtenberg B, et al. (2015) Genome analysis of Pseudomonas fluorescens PCL1751: a rhizobacterium that controls root diseases and alleviates salt stress for its plant host. PLOS ONE 10: 231. Link: https://bit.ly/3iwC0SK
- Lin H, Hu S, Liu R, Chen P, Ge C, et al. (2016) Genome Sequence of Pseudomonas koreensis CRS05-R5, an Antagonistic Bacterium Isolated from Rice Paddy Field. Front Microbiol 7: 1756. Link: https://bit.ly/3gkbSsk
- Rafikova GF, Korshunova TY, Minnebaev LF, Chetverikov SP, Loginov ON (2016) A new bacterial strain, Pseudomonas koreensis IB-4, as a promising agent for plant pathogen biological control. Microb 85: 333-341. Link: https://bit.ly/31CfOQQ
- Mohn WW, Wilson AE, Bicho P, Moore ER (1999) Physiological and phylogenetic diversity of bacteria growing on resin acids. Syst Appl Microbiol 22: 68-78. Link: https://bit.ly/38qX2gP
- Ngeranwa JJ, Gathumbi PK, Mutiga ER, Agumbah GJ (1993) Pathogenesis of Trypanosoma (brucei) evansi in small East African goats. Res Vet Sci 54: 283-289. Link: https://bit.ly/2Vz96aA
- Lopes MF, da Veiga VF, Santos AR, Fonseca ME, DosReis GA (1995) Activation-induced CD4+ T cell death by apoptosis in experimental Chagas' disease. J Immunol 154: 744-752. Link: https://bit.ly/3ilHG1J
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
