International Journal of Veterinary Science and Research
Evaluation of insulin resistance in overweight and obese dogs
Julio R Ramos S1 and Víctor Castillo2*
2University of Buenos Aires, Faculty of Veterinary Sciences, Chair of Small Animal Medical Clinic, Buenos Aires, Republic of Argentina
Cite this as
Ramos JR, Castillo V (2020) Evaluation of insulin resistance in overweight and obese dogs. Int J Vet Sci Res 6(1): 058-063. DOI: 10.17352/ijvsr.000055Prevalence of obesity in dogs has been increasing in the last decade, being the most common form of malnutrition and consequently has increased the appearance of metabolic diseases in this species. The objective of this study is to compare biochemical and endocrine profiles related to insulin resistance between lean and obese canine patients and to find similarities with the human metabolic syndrome. A total of 20 dogs were divided into two groups (lean and obese) of 10 dogs each, evaluating body mass index, HOMA-IR, HOMA-B, insulin, glucose, cholesterol, triglycerides, HDLc, LDLc, cortisol, T4total and freeT4. The results showed significant increase in BMI, HOMA-IR, insulin, glucose, cholesterol, HDLc, triglycerides and cortisol in obese patients, similar to metabolic syndrome described in humans.
Introduction
Obesity is the most common form of malnutrition in dogs and is defined as an increase of more than 15% of optimal body weight by the accumulation of adipose tissue [1]. There are predisposing factors such as: castration, sedentary lifestyle, high fat diets, and endocrine diseases such as hypothyroidism [2]. The prevalence of this disease has been increasing in recent years in companion animals with 30 to 40% of overweight pets and 5 to 20% are obese, and consequently increasing the occurrence of metabolic diseases such as dyslipidemia and insulin resistance or glucose intolerance [3,4]. Obese individuals present alterations in concentrations of fasting glucose, insulin, cortisol, lipids and lipoproteins, such as elevated cholesterol and triglycerides, as well as increased cholesterol-carrying lipoproteins: VLDL (very low density lipoprotein), low-density lipoprotein (LDL) with decreased levels of HDL (high-density lipoprotein) [5,6]. As in humans, obesity in dogs and cats is associated with a variety of metabolic dysfunctions [7].
Insulin resistance implies resistance to the effects of insulin on glucose uptake, metabolism or storage, and in obesity it is manifested by a decreased transport of glucose stimulated by insulin [8]. To compensate for this resistance, more insulin must be secreted, not being clear how it is increased. Possible mediators of this phenomenon are glucose and some hormones like cortisol, which in obese humans tend to increase its plasma and urinary concentration [9]. HOMAIR (Homeostatic Evaluation Model for Insulin Resistance), which is the ratio of fasting glucose and insulin concentrations to determine the degree of insulin resistance of the patient, [6,10,11] in the same way as the human, obese dogs show elevation in HOMA-IR indicating a decrease in insulin sensitivity in these patients [6,12,13].
Materials and methods
Population of study
A total of 20 dogs were divided into two groups of 10 dogs each. Owners authorized blood sample collection and participation in this study. The groups consisted of an age range between 1 year and 11 years, all of them being of different sizes. Visual body score (VBS) was used to assign them to each group. For this method, the fat that covers the ribs, under the back, around the base of the tail and ventrally along the abdomen was considered and assigned numbers from 1 to 5, with 3 being the ideal weight of the patient, 4 in overweight and 5 for obese patients [4,14,15]. The VBS evaluation was made by three observers. Patients who scored with numbers 4 and 5 for this method that corresponded to overweight and obese patients were assigned to the “obese group”, while patients who scored 3 were assigned to the “lean group”. In both groups, 5 males and 5 females were distributed equally between castrated and non-castrated females to avoid effects of castration on lipids metabolism [16,17].
Calculation of body mass index
Body mass index (BMI) was measured using formula described by [1]: Body mass index (BMI) = Body weight (BW) in kg/(height at shoulder in cm × length from occipital protuberance to base of tail in cm).
Biochemical studies in blood
In the two groups blood samples (12-hour fast) were obtained by venipuncture of the cephalic vein using 3 ml syringes with 23Gx1 NIPRO® needles and in Vacuette® MiniCollect tubes to analyze glucose, total cholesterol, HDL-cholesterol (HDLc), LDL-cholesterol (LDLc), and triglycerides. To avoid glucose consumption by the erythrocytes, the blood was collected in a separate tube with fluorinated anticoagulant, then centrifuged and separated to measure blood glucose. In another tube without anticoagulant blood was collected to measure total cholesterol, HDL cholesterol fraction and triglycerides after allowing the clot to form, centrifuging and separating the serum. These variables were measured using the VITROS 250® analyzer. The LDL cholesterol fraction was calculated using the Friedewald formula: LDL-cholesterol= Total cholesterol – (HDL-cholesterol + Tg/5) [18].
TSH must be between the reference range (<0.35ng/mL) in all dogs in order to be part of this study. Insulin, cortisol and thyroid hormones (total T4 and free T4) were evaluated (from the same blood sample obtained to measure the above-mentioned variables). Hormone samples were frozen at -30°C until processed. All were analyzed by chemiluminescence method (IMMULITE, 1000®, Siemmens corp). For cortisol the inter- and intra-assay coefficients of variation for cortisol were 8% and 5%, respectively; insulin, inter- and intra-assay coefficients of variation (canine performance) were 4.5% and 4.3%, respectively and sensitivity of 0.005 ng/ml; TSH, intraassay and inter-assay CV were 3% and 4.3%, respectively and the sensitivity was 0.03 ng/ml; total T4, intra-assay coefficient of variation was 3.1%, inter-assay coefficient of variation was 4.8%, Sensitivity 0,20 ug/dl; free-T4, intraa-ssay and inter-assay coefficient of variation were 4 and 3.4% respectively. Sensitivity 0,03 ng/ml. HOMAIR index for the assessment of insulin resistance and HOMAB were calculated using Oxfford’s HOMA Calculator version 2.2.3.
Statistical methodology
Distribution of the variables (parametric or non-parametric) was determined through D’Agostino-Pearson’s normality test. The obtained data were compared among the populations by means of the non-parametric test of Mann Whitney. Data are expressed as medians and minimum and maximum range. A correlation study was made by the Spearman correlation test. A value of P<0.05 was considered significant for all tests.
Results
Population of study
A total of 20 dogs divided into two groups: a group of dogs assigned as lean dogs (n = 10) and another group of dogs assigned as overweight and obese (n = 10). Each group had a homogenous distribution in terms of sex and reproductive status. Patients were in a range between 1 year and 11 years, which can be classified into three groups: young adults (patients between 1 and 3 years); adults (patients between 4 and 6 years old); and matures (patients older than 7 years). Breeds of the patients evaluated were in majority mongrel dogs, followed by Labrador Retrievers and Beagle.
Body mass index
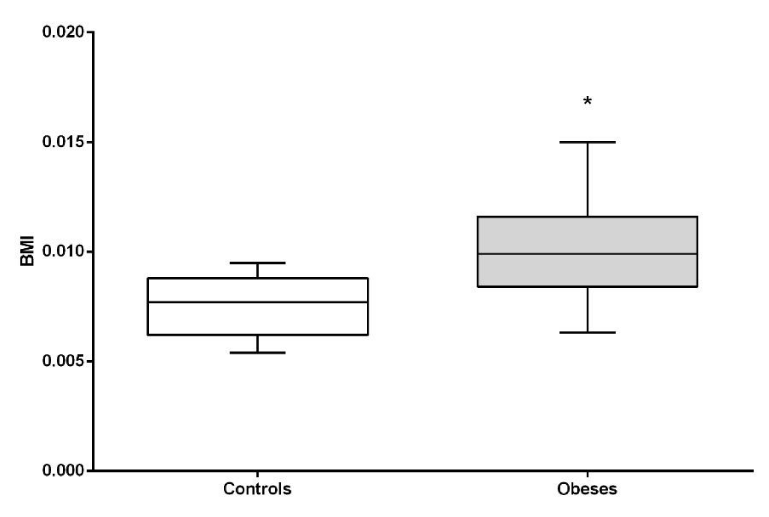
It was observed that BMI is significantly higher in the obese than in the normal weight (P <0.05). (Figure 1).
Blood glucose, insulin and HOMA-IR and HOMA-B index
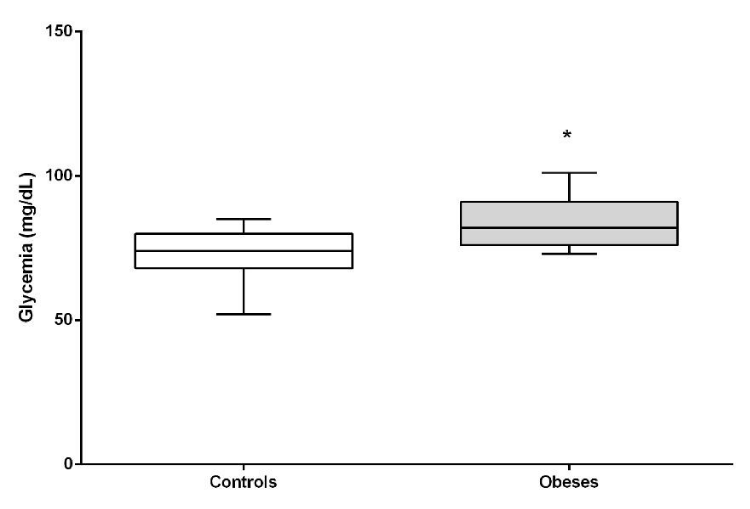
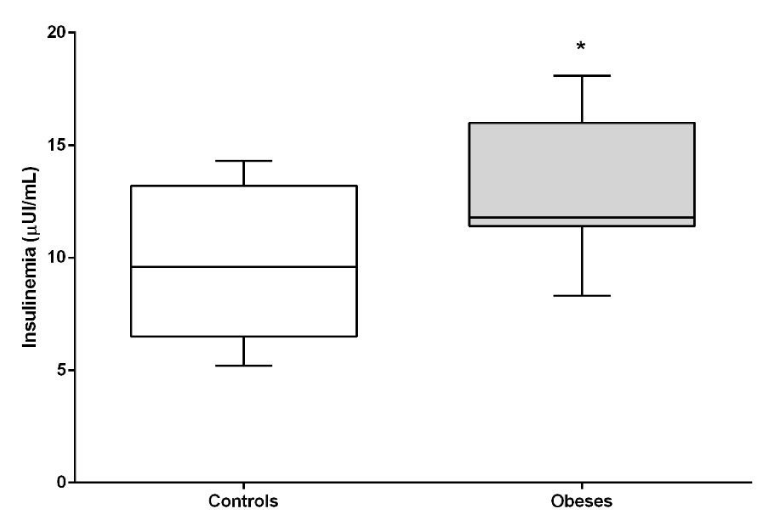
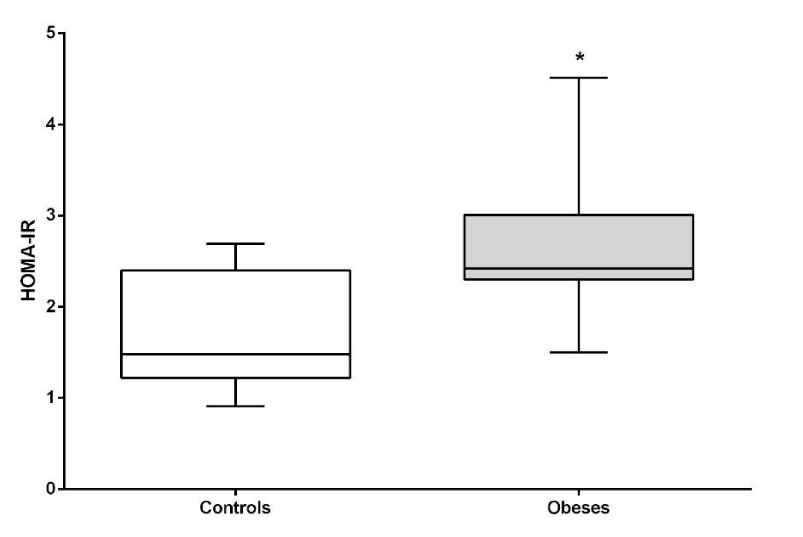
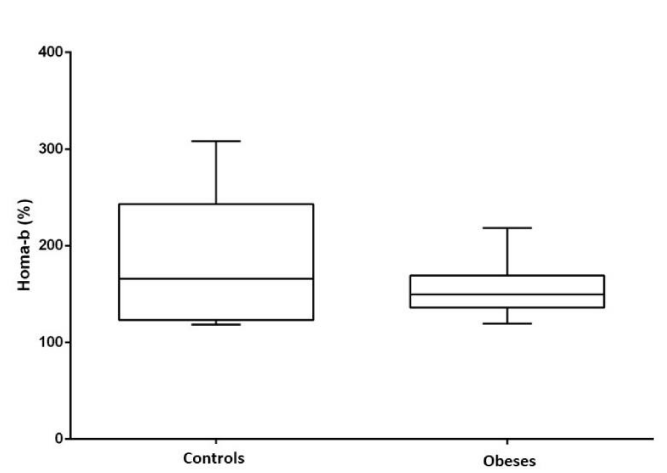
Both glycemia, insulin and HOMA-IR index were significantly higher in the obese group compared to lean dogs (P <0.05). HOMA-B (beta cell function) did not show differences between the groups (Figures 2-5).
Serum lipids
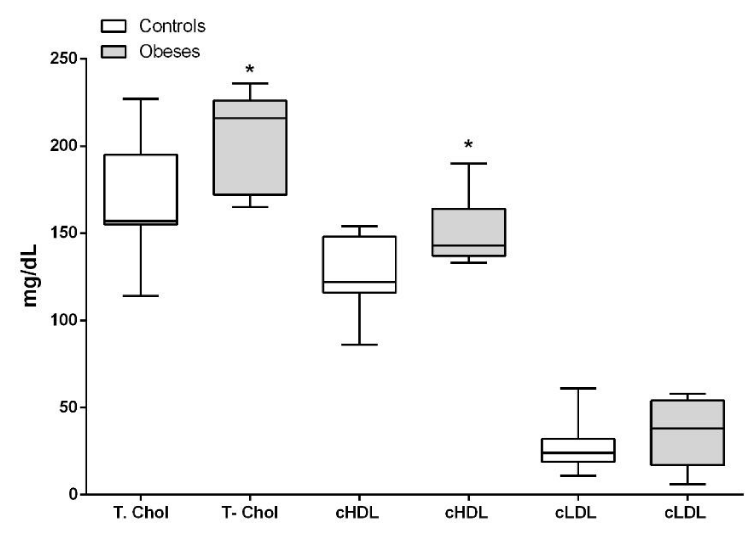
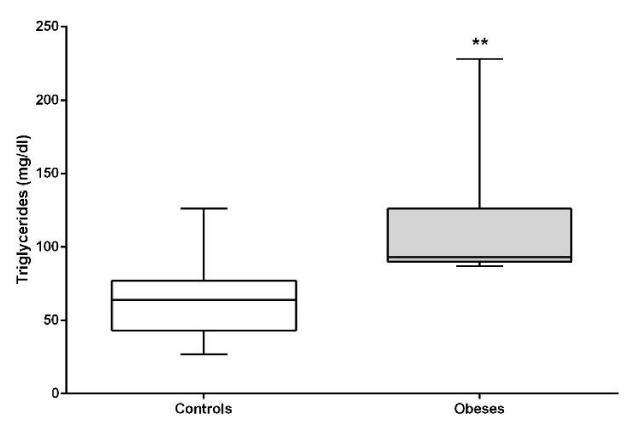
Total cholesterol (P<0.05), triglycerides (P<0.01) and HDL (P<0.05) were significantly higher in the obese compared to lean dogs, with no significant difference in LDL. (P>0.05). (Figures 6,7).
Cortisol and thyroid hormones
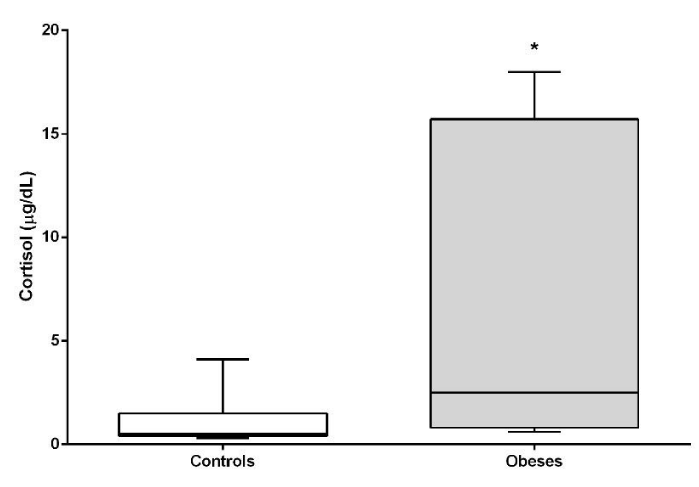
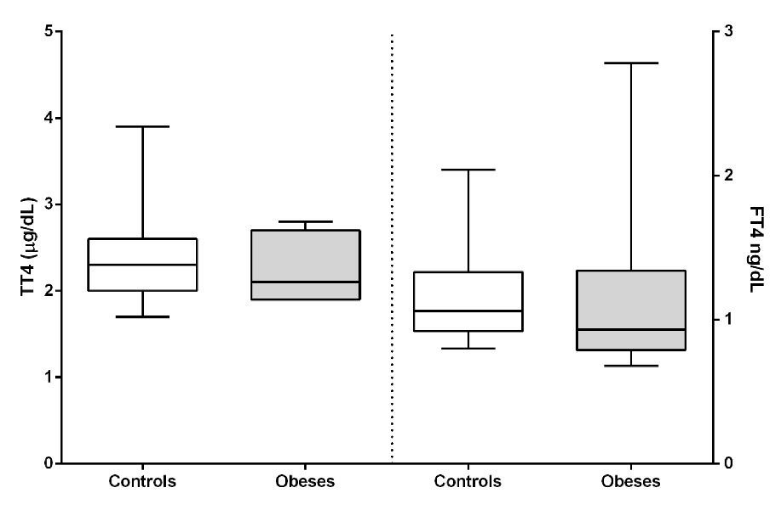
Cortisol concentrations were significantly higher (P<0.05) in the obese group. Thyroid hormones did not show significant differences between both groups (Figures 8,9).
Correlation analysis
VBS was correlated with the rest of the variables showing a significant correlation with all variables except cholesterol, its fractions and thyroid hormones (Table1).
Discusion
Currently obesity in the urban dog is considered an epidemic as in the human [19,20] caused by a positive energy balance and accumulation of adipose tissue with abdominal predominance (visceral fat). This type of adipocyte is described as smaller and unstable and is associated with metabolic disorders because leptin and adiponectin are affected, and for being susceptible to inflammation [21,22], this is the initial step for the development of Metabolic Syndrome that finally will end in diabetes mellitus without the adequate therapeutics (exercise, change of habits, adequate food).
In the present study we studied overweight and obese dogs. Using the method of morphometry to determine BMI [1], we compared it with VBS finding congruence in both methods since the group of obese patients had higher BMI than the control group. VBS is useful in clinical practice and would allow to have an approximate idea of what happens metabolically in the obese dog. This arises from the correlations observed in our study, where VBS correlated positively with glucose, triglycerides, insulin, HOMAIR and cortisol. Therefore, patients with VBS of 4 and 5 would be expected at the time of consultation to have impaired fasting glucose, higher concentrations of fasting triglycerides and hyperinsulinism with insulin resistance because of clinical obesity.
This study found significant alteration in fasting blood glucose concentrations in obese patients compared to lean dogs. These results are in accordance with similar previous studies [23] and in contrast to another research [12] who did not find differences between obese and lean dogs. Although the fasting glucose values of this study were within the reference range considered normal, the glycemia of the obese population was higher than lean dogs, which would indicate that the glycemic control is not adequate, as it has been described in human medicine in obese patients [24]. Some authors propose that blood glucose remains within its normal concentrations due to the hyperinsulinism that occurs in these patients, being a way to compensate and maintain the homeostasis of glucose at the expense of a greater synthesis and release of insulin [25].
Patients in the obese group presented hyperinsulinism coinciding with other studies, both in dogs and humans [12, 24, 25]. This is due to the compensatory response of the pancreatic beta cell to the increase in fasting glucose and greater difficulty of admission in peripheral tissues due to the lower insulin sensitivity [26], even at the expense of exhaustion and trigger the process of cellular apoptosis [27]. If beta cell overwork is not controlled and reversed, the risk of progressing to the state of diabetes mellitus is high [28].
The HOMA-IR index has been previously described in humans and dogs as an estimator of the insulin resistance state or peripheral sensitivity [6,10,29]. In our study, HOMA-IR was high in the obese group, indicating peripheral insulin resistance in this population. It is known that obesity causes insulin resistance by various mechanisms [8] and that this resistance can be reversed when the body weight decreases and the visceral adipose tissue decreases [12,30]. In this way, the peripheral sensitivity to insulin is restored by decreasing the adiponectin and the inflammatory cytokines responsible in part for the processes of insulin resistance. HOMA-B did not show any significant difference between the groups, this may suggest that beta-cells function remains stable despite peripheral insulin resistance.
The decrease in visceral adipose tissue is also important for the correct metabolism of lipids. Studies in humans and rats show a direct relationship between adipose tissue mass, triglyceride and total cholesterol levels [31]. We found in our work elevation in the concentrations of total cholesterol, HDL cholesterol and triglycerides. Similar studies [19], found alterations in total cholesterol only in obese canine patients. These same alterations in total cholesterol and triglycerides were described in obese felines who also had decreased concentrations of adiponectin associating it intimately with triglycerides with insulin resistance [32], the same thing that has long been described in human medicine [33,34]. Some researchers also found alterations in cholesterol and triglycerides in obese dogs together with hyperinsulinism and higher plasma glucose concentrations, postulating that these anomalies are similar to what was described in humans as Metabolic Syndrome, so it postulates that 20% of dogs suffer from this syndrome which the author calls obesity- related metabolic dysfunction in dogs [35].
Other studies conducted in obese humans have also determined significant alterations in plasma lipid concentrations mainly in triglycerides and HDL [36,37] and significant alterations in total cholesterol, triglycerides, HDL and LDL [38]. The HDL cholesterol is high since it corresponds to 70% of total cholesterol, however, in terms of LDL there was no significant increase in our work.
The concentrations of thyroid hormones were normal, which indicates that these dogs did not suffer from hypothyroidism, so obesity is not attributable to this cause but to the fact of sedentary lifestyle and overfeeding. On the other hand it is clearly seen that obesity does not affect, at least as regards thyroxine concentrations, the function of the thyroid gland as it does with the endocrine pancreas. It would be interesting to further analyze the thyroid gland since it has been described that insulin resistance affects its functionality [39].
In humans, cortisol has been implicated as a physiopathological marker in idiopathic obesity, however, the concentrations of this circulating hormone are not always high [40]. In this work, obese dogs show an increase in serum cortisol levels. Given this, several explanations have been previously postulated in studies conducted in humans for the increase in cortisol secretion in obese patients: the first indicates that the increase in circulating cortisol levels is proportional to the increase in body mass [41]; a second explanation indicates a primary neuroendocrine abnormality causing an increase in central stimulation of the secretion of corticotropin-Releasing Hormone (CRH), corticotropin (ACTH) and cortisol [42] and a third postulates that there is an increase in the peripheral metabolism of cortisol with compensatory changes of the hypothalamic-pituitary-adrenal axis [41]. Local control of glucocorticoid production is carried out by the enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), which converts inactive cortisone into cortisol. This enzyme is widely expressed in several tissues, including visceral adipose tissue. Studies in humans have shown that obese patients express more 11β-HSD1 in fat tissue compared to non-obese subjects [20,43]. Recently it was possible to verify in dogs with Cushing’s syndrome (they have a large amount of visceral fat) that this enzyme is also more expressed [44]. This higher expression would explain the significant elevation of basal cortisol in obese dogs in this study. This excess of cortisol promotes hepatic gluconeogenesis, countering the action of insulin in the hepatocyte [45] and being one of the causes of the increase in fasting blood glucose. On the other hand, it should be considered that cortisol will affect the action of insulin in peripheral tissues and promotes lipolysis with an increase in free fatty acids and triglycerides [46]. In this way, there are similarities between the metabolic syndrome that incorporates central obesity, dyslipidemia and insulin resistance with hypercortisolism.
In conclusion, obese dog has metabolic syndrome, and this is evidenced by the combination of insulin resistance, hyperglycemia, hyperinsulinemia and hypertriglyceridemia, being sustained by hypercortisolism that creates a vicious circle. Fasting hypertriglyceridemia is an indicator of insulin resistance, therefore if we consider these animals with high triglycerides and higher blood glucose levels, and based on the HOMAIR index, obese dogs in our study show insulin resistance.
- Mawby DI, Bartges JW, d’Avignon A, Laflamme DP, Moyers TD, et al. (2004) Comparison of Various Methods for Estimating Body Fat in Dogs. J Am Anim Hosp Assoc 40: 109-114. Link: https://bit.ly/2zFffKw
- Laflamme DP (2006) Understanding and managing obesity in dogs and cats. Vet Clin North Am Small Anim Pract 36: 1283–1295. Link: https://bit.ly/2TNe1UE
- Veiga APM, Price CA, Oliveira ST, Dos Santos AP, Campos R, et al. (2008) Association of canine obesity with reduced serum levels of C-reactive protein. J Vet Diagn Invest 20: 224-228. Link: https://bit.ly/2zJEM5j
- Witzel AL, Kirk CA, Henry GA, Toll PW, Brejda JJ, et al. (2014) Use of a novel morphometric method and body fat index system for estimation of body composition in overweight and obese dogs. J Am Vet Med Assoc 244: 1279-1284. Link: https://bit.ly/3ch5Hme
- Jordan E, Kley S, Le NA, Waldron M, Hoenig M (2008) Dyslipidemia in obese cats. Domest Anim Endocrinol 35: 290-299. Link: https://bit.ly/2TNWZpc
- Verkest KR, Fleeman LM, Rand JS, Morton JM (2010) Basal measures of insulin sensitivity and insulin secretion and simplified glucose tolerance tests in dogs. Domest Anim Endocrinol 39: 194-204. Link: https://bit.ly/2XeKmFG
- Zoran DL (2010) Obesity in dogs and cats: a metabolic and endocrine disorder. Vet Clin North Am Small Anim Pract 40: 221-239. Link: https://bit.ly/2yHSxRx
- Kahn BB, Flier JS (2000) Obesity and insulin resistance. J Clin Invest 106: 473-481. Link: https://bit.ly/2XFiWrR
- Abraham SB, Rubino D, Sinaii N, Ramsey S, Nieman LK (2013) Cortisol, Obesity, and the Metabolic Syndrome: A Cross-Sectional Study of Obese Subjects and Review of the Literature. Obesity 21: 105-117. Link: https://bit.ly/2M8Qj0J
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412-419. Link: https://bit.ly/2zyS9FH
- Graciela BS, Wolfthal DL (2008) Valores de corte para índices de insulinorresistencia, insulinosensibilidad e insulinosecreción derivados de la fórmula HOMA y del programa HOMA2: Interpretación de los datos. Rev Argent Endocrinol Metab 45: 03-21. Link: https://bit.ly/2Aj3lGo
- Mannucci E, Ognibene A, Cremasco F, Bardini G, Mencucci A, et al. (2000). Glucagon‐like peptide (GLP)‐1 and leptin concentrations in obese patients with Type 2 diabetes mellitus. Diabetic Medicine 17: 713-719. Link: https://bit.ly/2XEJg5b
- German, AJ, Hervera M, Hunter L, Holden SL, Morris PJ, et al. (2009) Improvement in insulin resistance and reduction in plasma inflammatory adipokines after weight loss in obese dogs. Domest Anim Endocrinol 37: 214–226. Link: https://bit.ly/36GWZfX
- Thatcher CD, Hand MS, Remillard RL, eds. (2010) Small Animal Clinical Nutrition. 5th ed. Topeka: Mark Morris Institute 501-435.
- Jeusette I, Greco D, Aquino F, Detilleux J, Peterson M, et al. (2010). Effect of breed on body composition and comparison between various methods to estimate body composition in dogs. Res Vet Sci 88: 227–232. Link:https://bit.ly/2ZMrQGr
- Edney AT, Smith PM (1986) Study of obesity in dogs visiting veterinary practices in the United Kingdom. Vet Rec 118: 391-396. Link: https://bit.ly/2ZNdaaf
- McGreevy PD, Thomson PC, Pride C, Fawcett A, Grassi T, et al. (2005) Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Vet Rec 156: 695-702. Link: https://bit.ly/2XetXRV
- Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clinical chemistry 18: 499-502. Link: https://bit.ly/2MbRixi
- Jeusette IC, Lhoest E, Istasse LP, Diez MO (2005) Influence of obesity on plasma lipid and lipoprotein concentrations in dogs. Am J Vet Res 66: 81-86. Link: https://bit.ly/2MbRA7m
- Baudand R, Arteaga E, Moreno M (2010) El tejido graso como modulador endocrino: Cambios hormonales asociados a la obesidad. Rev Med Chile 138: 1294-1301. Link: https://bit.ly/3dguFU9
- Trayhurn P (2005) Adipose tissue in obesity — an inflammatory issue. Endocrinology 146: 1003-1005. Link: https://bit.ly/2zH5ADo
- German AJ, Ryan VH, German AC, Wood IS, Trayhurn P (2010) Obesity, its associated disorders and the role of inflammatory adipokines in companion animals. Vet J 185: 4-9. Link: https://bit.ly/2Mcflfk
- Verkest KR, Rand JS, Fleeman LM, Morton JM (2012) Spontaneously obese dogs exhibit greater postprandial glucose, triglyceride, and insulin concentrations than lean dogs. Domest Anim Endocrinol 42: 103-112. Link: https://bit.ly/2zFmrX2
- Haffner SM, Stern MP, Mitchell BD, Hazuda HP, Patterson JK (1990). Incidence of type II diabetes in Mexican Americans predicted by fasting insulin and glucose levels, obesity, and body-fat distribution. Diabetes 39: 283-288. Link: https://bit.ly/2MbSD7i
- Verkest KR, Fleeman LM, Rand JS, Morton JM (2011) Evaluation of beta-cell sensitivity to glucose and first-phase insulin secretion in obese dogs. Am J Vet Res 72: 357–366. Link: https://bit.ly/2MasLZo
- Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, et al. (2001) Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 50: 1126–1133. Link: https://bit.ly/3c7SZGw
- Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, et al. (1998) Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes 47: 358-364. Link: https://bit.ly/36Ikp4H
- Weyer C, Bogardus C, Mott DM, Pratley RE (1999) The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Investig 104: 787–794. Link: https://bit.ly/2B88y44
- Radziuk J (2000) Insulin sensitivity and its measurement: structural commonalities among the methods. J Clin Endocrinol Metab 85: 4426-4433. Link: https://bit.ly/3gDklrE
- Henriksen EJ (2002) Exercise Effects of Muscle Insulin Signaling and Action Invited Review: Effects of acute exercise and exercise training on insulin resistance. J Appl Physiol 93: 788–796. Link: https://bit.ly/2XJxiqS
- Krause BR, Hartman AD (1984) Adipose tissue and cholesterol metabolism. J Lipid Res 25: 97-110. Link: https://bit.ly/2TQZmHY
- Hatano Y, Mori N, Asada M, Mori A, Yamamoto I, et al. (2010) Hypertriglyceridemia with increased plasma insulin concentrations in cats. Res Vet Sci 88: 458-460. Link: https://bit.ly/3gC5kWI
- McKeigue PM, Shah B, Marmot MG (1991) Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 337: 382-386. Link: https://bit.ly/2TR1S0J
- Williams DP, Going SB, Lohman TG, Harsha DW, Srinivasan SR, et al. (1992) Body fatness and risk for elevated blood pressure, total cholesterol, and serum lipoprotein ratios in children and adolescents. Am J Public Health 82: 358-363. Link: https://bit.ly/2XHt7vR
- Tvarijonaviciute A, Ceron JJ, Holden SL, Cuthbertson DJ, Biourge V, et al. (2012) Obesity-related metabolic dysfunction in dogs: a comparison with human metabolic syndrome BMC Veterinary Research 8:147. Link: https://bit.ly/3gvASh4
- Barja S, Barrios X, Arnaiz P, Domínguez A, Villarroel L, et al. (2013) Niveles de lípidos sanguíneos en escolares chilenos de 10 a 14 años de edad. Nutr Hosp 28: 719-725. Link: https://bit.ly/2AkkEa5
- Domínguez LR, Díaz ME, Ruiz V, Hernández H, Herrera V, et al. (2013) Relación entre lípidos séricos y glucemia con índice de masa corporal y circunferencia de la cintura en adolescentes de la secundaria básica Protesta de Baraguá-Cuba. Perspectivas en nutrición humana 15: 135-148. Link: https://bit.ly/3dfwTTJ
- Romero E, Campollo O, Celis A, Vásquez EM, Castro JF, et al. (2007) Factores de riesgo de dislipidemia en niños y adolescentes con obesidad. Salud Pública Mex 49: 103-108. Link: https://bit.ly/2Xff8yp
- Wesche MF, Wiersinga WM, Smits NJ (1998) Lean body mass as a determinant of thyroid size. Clin Endocrinol 48: 701-706. Link: https://bit.ly/2yNFaiW
- Rask E, Olsson T, Söderberg S, Adrew R, Livingstone D, et al. (2001) Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab 86: 1418-1421. Link: https://bit.ly/36H5He8
- Strain GW, Zumoff B, Strain JJ, Levin J, Fukushima DK (1980) Cortisol production in obesity. Metabolism 29: 980-985.
- Björntorp P, Holm G, Rosmond R (1999) Hypothalamic arousal, insulin resistance and Type 2 diabetes mellitus. Diabet Med 16: 373-383. Link: https://bit.ly/3eulfEL
- Iwasaki Y, Takayasu S, Nishiyama M, Tsugita M, Taguchi T, et al. (2008) Is the metabolic syndrome an intracellular Cushing state? Effects of multiple humoral factors on the transcriptional activity of the hepatic glucocorticoid-activating enzyme (11β-hydroxysteroid dehydrogenase type 1) gene. Molecular and Cellular Endocrinology 285: 10–18. Link: https://bit.ly/2M9vqCv
- Miceli DD, Castillo V (2017) Alteración de la expresión de la 11β-hidroxideshidrogenasa tipo 1 (11β-HSD 1) y disminución del péptido glucagón símil-1 (GLP-1) en perros con Síndrome de Cushing. Tesis doctoral, Universidad de Buenos Aires.
- Wake DJ, Walker BR (2004) 11β-Hydroxysteroid dehydrogenase type 1 in obesity and the metabolic syndrome. Mol Cell Endocrinol 215: 45–54. Link: https://bit.ly/2ZKBFVz
- Andrews RC, Walker BR (1999) Glucocorticoids and insulin resistance: old hormones, New targets. Clin Sci 96: 513-523. Link: https://bit.ly/2XdlgHp
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
