International Journal of Veterinary Science and Research
Prevalence, associated risk factors and species identification of lung worm infection in sheep in Dangla district, Western Amhara, North West Ethiopia
Department of Veterinary Medicine, School of Veterinary Medicine, Wollo University, Dessie, Ethiopia
Author and article information
Cite this as
Habte D, Simeneh A (2019) Prevalence, associated risk factors and species identification of lung worm infection in sheep in Dangla district, Western Amhara, North West Ethiopia. Int J Vet Sci Res. 2019; 5(2): 076-085. Available from: 10.17352/ijvsr.000045
Copyright License
© 2019 Habte D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Cross-sectional study was conducted from November 2014 to May 2015 to determine lungworm prevalence, risk factors associated with lungworm infection and identifi cation of species of lungworm in sheep in Dangla district, Northwestern Ethiopia.
AGDP: Agricultural Gross Domestic Product; AZARDO: Awi Zone Agriculture and Rural Development Office; CSA: Central Statistical Authority; DACA: Drug Administration Control Authority of Ethiopia; EARO: Ethiopian Agricultural Research Organization; FAO: Food and Agricultural Organization ILCA: International Livestock Center for Africa; NMSA: National Meteorology Service Agency; SPSS: Statistical Package for Social Science
Introduction
Sheep are the most numerous of man’s domestic livestock and are especially important in the more extreme climates. Their small size, high productive capacity and rapid growth rate make sheep a more flexible short term form of investment than cattle [1]. Of the world’s 1,614 million or 65% of sheep are located in developing countries. In Africa they are noted for their ability to convert low opportunity meat, milk, fiber, manure and hides [2,3].
In Ethiopia, agriculture is the mainstay of the country and also the major resources of employment and income, about 80% of the population live in the rural areas and are primarily engaged in agriculture and related activities. Thus, agriculture directly or indirectly forms an important component of the livelihood of more than 60 million people in the country. In Ethiopia, livestock contribute about 30%-35% of the agricultural Gross Domestic Product (GDP) and more than 85% of farm cash income [4].
In terms of live stock population, Ethiopia stand first in Africa and 10th in the world in live stock population. The domestic animal population of the country is estimated to be more than 38,749,320 cattle, 18,075,580 sheep, 14,858,650 goats, 456,910 camels, 5,765,170 equines and 30,868,540 chickens with livestock ownership currently contributing to the livelihoods of an estimated 80% of the rural population [5].
In Ethiopia, sheep are the dominant livestock providing up to 33% of cash income and 23% of food substance value obtained from livestock production. Sheep play a vital role as sources of meat, milk and wool for smallholder keepers in different farming systems and agro-ecological zones of the country [6]. They are also sources of foreign currency. Sheep and goat contribute a quarter of the domestic meat consumption; about half of the domestic wool requirement, 40% of fresh skins and 92% of the value of semi- processed skin and hide exports to abroad. It is estimated that 1,078,000 sheep are used in Ethiopia for domestic consumption annually. There is also a growing export market for sheep meat in the Middle Eastern Gulf States and some African countries. At optimum off take rates, Ethiopia can export 700,000 sheep’s meat annually, and at the same time supply, 1,078,000 sheep’s for the domestic market [7].
Unlike the large population and importance of sheep in the country their productivity is low. This low productivity is a reflection of diseases, poor nutrition, poor animal production system and general lack of veterinary care [8]. Livestock diseases are widely distributed and are one of the major causes of livestock mortality and sub-optimal productivity in all agro-ecological zones of the country. Respiratory diseases resulting from helminthes parasites are of great economic concern in sheep production in the low lands and highlands of Ethiopia where sheep are important livestock units [9].
Dictyocaulidae and certain Metastrongylidae are known to exist in East Africa (Ethiopia, Kenya and Tanzania) and the South Africa. In lowland and highlands area of Ethiopia, respiratory lungworm parasites are the most common cause of high morbidity and mortality rates of sheep [6,10].
Lungworm infection is infection of lower respiratory tract, resulting in bronchitis or pneumonia or both. Any of several parasitic nematode including; Dictyocaulus viviparous in cattle; Dictyocaulus arnifieldi in donkeys and horses; Dicyocaulus filaria, Protostrongylus rufescence and Mullerius capillaris in sheep and goats; Metastrongylus apri in pigs; filriodes (oslerus osleri) in dogs and Aelurostrongyllus abstrusus in cats; other lung worm infection occur but less common [11]. These lungworms particularly Dictyocaulus filaria can suppress the immunity of the respiratory tract and causes death, poor weight gain or loss of body weight as well as greatly affects the potential productivity of sheep in the areas where it is prevalent [12]. Prevention and Control of these parasites is therefore essential for releasing the potential of sheep production.
For proper control to be carried out knowledge of parasitic diseases and their dynamics must be understood to lay down rigid rules for their control which are applicable to all regions. For this reason, a study of epidemiology of each parasitic disease should be limited to small areas [13]. In order to investigate a lungworm control strategy at local and regional level, further and detailed investigation on epidemiology and importance of lungworm infections with respect to associated risk factors is necessary. but there are very limited studies that have been conducted so far by [14], with Prevalence of 13%, [15], with 20%, and [16], with 17.5% in and around Bahir-Dar which has the same agro-ecological climate with Dangla where no research have been done on the prevalence of lungworm infections in sheep. Therefore, the study was designed with the objectives to:-
¬ Determine the prevalence of lungworm infection in sheep
¬ Determine the associated risk factors with the occurrence of lungworm infection in the area
¬ Identify the species of the respiratory helminthes circulating in study area
Literature review
Lung worm infection of sheep: The common name of lungworm infection is varminous bronchitis or verminous pneumonia. Verminious pneumonia is a chronic and prolonged lung infection of sheep. It is characterized clinically by respiratory distress and pathologically by bronchitis and bronchopneumonia and caused by nematode parasite. Lungworms are parasitic nematode of the order strongylida that infest the lungs of vertebrates. It is infection of lower respiratory tract resulting in bronchitis, or pneumonia or both, by any of several parasitic nematodes [17].
Etiology: Lungworms of sheep are nematodes that belong to phylum Namath helminthes commonly named as round worms classified under the supper family Trichostronglylodae and Metastrongylidea which cause lung worm infection in sheep. Dictyocaulidae species are located in the bronchial tree in lung while protostrongylidae species are located in lung parenchyma mainly in the form of brood and worm nodules and for those M. capillarises found in Alveoli [18]. The common causes of verminous pneumonia in sheep are Dictyocaulus filaria (D. filaria), Protostrogylus rufescens (P. rufescens) and Mullerias Capillaries (M. capillaries). D. filarial is belonged to super family Trichostrongyloidae, and, Protostrongylus rufescens and Mullerius capillaries are belonged to the family Metastrongyloidae. D. filaria predominant in most outbreaks [19]. Lungworm infestation in different species caused by different nematodes and the most common lungworms found in animals are shown in (table 1).
Morphology: Member of the family Dictoycaulidea are unusual among the Trichostrongyl in that they Parasitizes the lungs especially the bronchi of the diaphragmatic lobes and trachea. The larvae of D. filaria measures 350-380µm. It has cuticular knob at the anterior extremity, straight tail and intestinal cells contain characteristic dark granules. The adult male, D. filaria is 3-8 cm long, while the female is 5-10 cm long. The worm has milk white color and the intestine shows as dark line. The mouth is surrounded by four lips and the mouth opening leads in to small bucal capsule. The males have a prominent copulatory bursa [20].
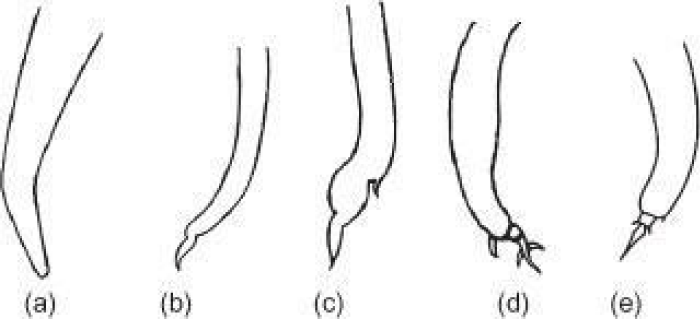
The larvae of P. rufescens and M. capillaries are smaller in size than D. filaria. These larvae are differentiated by their characteristic features at the top of their tail. Larva of P. rufescens has a wavy outline at tip of its tail but devoid of dorsal spine; on the other hand M. capillaries has kinked tail, undulating tip and dorsal spine [21].
Mullerius capillaris occur in alveolar ducts, small bronchioles and lung parenchyma of sheep [13]. The male measures 11-26 mm in length and female18-30mm, having spirally willed tail but no bursa on the female. There is sub-terminal valve and small cuticualr thickening on its border [21,22].
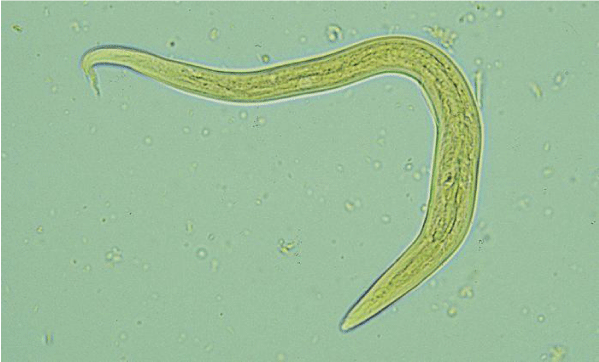
Protostrogylus rufescens occurs in small bronchioles of sheep. Both male and female are filari form and reddish in color while differ in their length (the male measures 16-28 mm and the female is 25-35 mm in length). In addition, the female worm identified by their conical tail and terminates in a small point and the vulva opens near the anus (Figure 1) [19,22].
Epidemiology: Lungworms are widely distributed throughout the world but they are particularly common in countries with temperate climate and in highlands of tropical and subtropical countries. In temperate areas, the epidemiology of the parasite is highly influenced by the survival of overwintered larvae on pasture and the role of the ewe and doe as a carrier are significant factors in the persistence of infection on pasture from year to year in endemic areas. Pasture infectivity is related to rainfall which stimulates activity of both larval and mollusk [15].
The larva of D. filaria have a considerable ability to resist cold climates, the larvae require moisture for development. D. filaria has a worldwide distribution and cause serious losses but an outbreak of infection often occurs with high mortality in sheep in temperate areas of the world. The larvae of D. filaria can withstand moderately dry condition for few days, but in moist condition for several months and are fairly resistance to low temperature [23].
Animals 2 to 18 months old have higher incidence than do other age groups. Many fifth stage larvae are inhibited in their development during winter in the lungs of older animals but resume the processes in spring [24].
Moisture is essential for the survival and development of the larvae. The larvae are active at moderate temperature of 10-210c. Infection of animals with lung worm have a very wide distribution depending on surrounds especially when the environment is established by the presence of long herbage of free water under optimal conditions the larvae can persist for over one year [21]. They reach the infective stage in 6 or 7 days. The primarily source of infection is infected pasture and water. The third stage (L3) larvae may survive for several months on pastures. Small number of adult worm can survive in the bronchi of infection animals particularly year lings [25]. Infective L3 can migrate from the faeces without the need for fungal dispersion. It is likely that only two cycles of the parasite occur during each grazing season. In warmer climates, where conditions are often unsuitable for larval survival, the carrier animal is probably a more important source of pasture contamination and outbreaks of disease in young susceptible animals are most likely to occur after a period of prolonged rain around weaning. In ewes, it is likely that the parasites are present largely as hypobiotic larvae in the lungs during each winter and mature in the spring. Development to the L3 only occurs during the period from spring to autumn. In lambs, patent infections first occur in early summer, but the heaviest infections are usually seen in autumn [20,21].
Muellerius is by far the commonest genus of sheep lungworm. In many temperate areas such as Britain, the eastern states of the USA and the winter rainfall regions of Australia, almost all sheep carry the infection; the extensive distribution and high prevalence are partly attributable to its wide range of intermediate hosts and the ability of larvae to overwinter in the mollusks. Additional factors which play a part in ensuring the endemicity of these worms are, first, the ability of the L1 to survive for months in faecal pellets and secondly, the persistence of the L3 in the intermediate host for the lifetime of the mollusk. Wild small ruminants are frequently heavily infected and could transmit protostrongylids to grazing sheep under some management systems [19,21]. The larvae of M. capillaris can resist fair amount of drying, are most active at relatively low temperature (17-27οc), and not killed by freezing. The infective larvae can also live up to a week after death of snail. Large worm burden or heavy infection not common in old sheep, as repeatedly infected animal becomes resistant [17].
Protostrongylus, whose intermediate host range is restricted to certain species of snail, has a lower prevalence, though its geographic range is just as wide as Muellerius. Additional factors which play a part in ensuring the endemicity of these worms are, first, the ability of the L1 to survive for months in faecal pellets and secondly, the persistence of the L3 in the intermediate host for the lifetime of the mollusc [21,23]. Also important in this respect are long period of potency and the apparent inability of the host to develop acquired immunity so that adult sheep have the heaviest infection and highest prevalence [17,19].
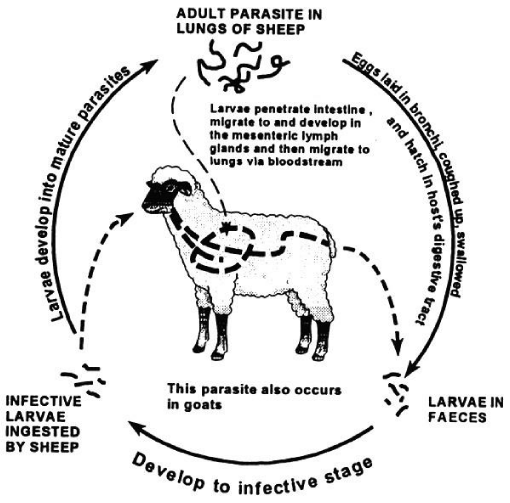
Life cycle: The life cycle of D. filaria is direct (Figure 2). The eggs hatch in the lung, but are usually coughed up and swallowed and hatch while they pass through the alimentary tract of the host. Some eggs may be expelled in the nasal discharge or sputum. The first stage larvae in the feces contain numerous brownish food granules in the intestinal cell, up in which the free stage larva depends on. The larva reaches the second stage after 1-2 days, but do not cast the old cuticle until the third or infective stage reached.
The first is then cast while the second is retained for protection. The larva reaches infective stage 6-7 days at 270c; the larvae at this stage are not very active and have weak geotropism [21,23,26]. The development to reach infective stage (L3) may take longer than 6-7days depending on ambient temperature and humidity. Desiccation rapidly kills the larva where as moderate temperature and high humidity will enhance survival of larva [27].
Infection of host occur per os, the parasite reach the lung by migrating through the lymphatic and circulatory system after penetrating the intestinal wall. Within three days, they develop and perform the third ecdysis (thesaurus) in the mesenteric lymph glands about four days after infection. Male and female distinguished at this stage; they are arrested in the capillaries and break through into air passages. Development to maturity takes about four weeks. Mature parasites develop in bronchi or bronchiole [26]( Figure 2).
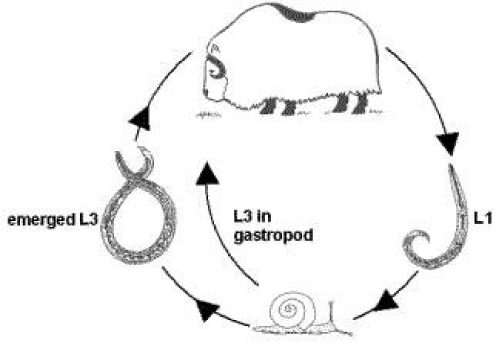
The metastrongylus species have indirect life cycle (Figure 3). They require a land snail as intermediate host since larva can only mature in an intermediate host [21,27]. The eggs develop in lung of host and the first stage larvae are passed in the faces. For further development they requires snail intermediate host, into which they penetratethrough foot or by which they are swallowed. The developments to the infective stage require 12-14 days and two ecdysis are performed. The final host becomes infected by swallowing the snail with its food and the larvae pass to the lung of the host via mesenteric lymph gland in which 3rd ecdysis takes place [21,23].
The adult M. capillaries live in alveolar parenchyma and sometimes called nodular lungworm [29]. In Protostrongylus rufescens transplacental transmission occurs and larvae can be found in the liver and lung of fetus and newborn lambs (Figure 3) [21,23].
Pathogenesis: The pathogenic effect of lungworm depends on their location within respiratory tract, the number of infected larvae ingested, the animal immune status, the nutritional status and age of the host [21,27,30].
During early stage of dictyocaulus infection (prepatent phase) the small bronchioles blocked by exudates, which obstructs the air way and this may result collapse of lung tissue. Larvae migrating through alveoli produce inflammatory response and this response is the case for blockage of bronchi by exudates. The bronchi contain fluid and immature worms, later adult worms and exudates they produce also blockage of bronchi. Secondary bacterial pneumonia and concurrent viral infection are often complication of dictyocaulosis [21,29]. Inflammatory process spreads to the surrounding peri- bronchial tissue and exudates pass back in bronchioles and alveoli, causing atelectasis and catarrhal pneumonia [21,25].
The smallest importance of Protostrongylus derives from the nature of lesion which they produce. Thus tend to be local small and accompanied by a swift local reaction of the host which limits their extension. In P. rufescens infection, the affected alveolar and bronchiolar epithelium is desquamated, blood vessels are occluded and infiltration with round cell and proliferation of connective tissue takes place in the area. The result is a small focus of lobular pneumonia roughly conical in shape and yellowish gray in color [21,23].
It is suggested that the larval stage of M. capillaris migrated through the wall of small intestine, the resulting damage may predispose to enterotoxaemia. It is possible that parasite is more harmful than popularly believed. Its effect is a triggering mechanism in Mycoplasma ovipneumonia infection [17,21]. The adult nematode in bronchi case bronchitis emphysema, pulmonary edema and secondary infection are common complication in severe case. After 2-3 months all or most of adult worms are expelled [21,27].
Clinical findings: The clinical manifestation of verminous pneumonia varies with the number of infecting worms. The most common sign are coughing and unthriftness which in endemic areas, is usually confined to young animals. In more severe cases dyspnoea and tenacious nasal discharge are also present. The sign may be accompanied by diarrhea or anemia due to concurrent intestinal trichostrongylosis [19,21,27]. A typical sound ‘husk-hoose’ (hoose husk disease) is produce due to occlusion of passage of the bronchi. Increasing of Body temperature may occur when there is secondary bacterial complication [26].
Necropsy findings: Necropsy in parasitic bronchitis is associated with progressive developing bronchitis and pneumonia. Several species of lungworm may cause pathological change and the post mortem examination of the lung may reveal sign of pneumonia, pleuritis, swelling and nodules. Adult Dictyocaulus species cause bronchitis and tracheitis. Whereas P. reufusen and M. capillaris cause alveolitis [21,27].
Antigenecity and development of immunity: Both cellular and humeral antibody reactions are observed following experimental or natural infection with lungworm infections. Lambs infected with D. filaria give a marked skin reaction to extracts of the parasite. With regard to cellular reaction high eosiniphilia counts are typical feature of lung worm infection [21]. But in the absence of re-infection the immunity may decrease rendering the animal susceptible again [21,31].
Diagnosis: Diagnosis of lungworm infection is based on clinical sign, epidemiology, and presence of first stage larvae in feces and necropsy of animal. In the same herd or flock larvae are not found in the feces of animals in the pre patent or post patent and usually not in the reinfection phenomena or may be few in number in early stages of an outbreak. Apart from epidemiological history, the clinical sign of coughing is indicative of parasitic bronchitis due to D. filaria [21,25].
First stage larvae or larvae eggs can be recovered using most fecal flotation with appropriate salt solution. A convenient method for recovering larvae is a modification Barman technique in which feces (5gm) are wrapped in tissue or cheese cloth and suspended or placed in water contained in conical flask. The water at the bottom of the flask is examined for larvae after 24 hours, in heavy infection larvae may present as many in number that is moving under low power microscopy [27,32]. Adults D. filaria are easily found in trachea and bronchi at necropsy but finding immature stage usually necessities dissecting pulmonary tissue and either allowing it to set in physiological saline or using Barman technique with physiological saline [21,27].
For most disease of helminthes origin, it is usually much easier to arrive the diagnosis by examining feces or larvae. For this reason, immunodiagnostic methods have not been very well practiced in diagnosis of helmenthosis including lungworm infection. However, some of experimental studies conducted in small ruminant have shown good results in detecting D. filarial infection using serological test. Trail conducted using Enzyme linked immune assay (ELISA) test on experimentally infected animals have shown a comparable result withy Radio immune assay (RIA) and antibodies were detected as early as 10th days after infection [21,31] ( Figures 4,5).
Treatment: For sheep lung worm infection Ivermectin 0.02mg/kg, oxfendazole 5mg/kg, fenbendazole 5mg/kg, levamisole 2.5mg/ kg, albendazole 4-8mg/kg and febantel 5-10mg/kg are the drugs of choice. These antihelmenthics have high activity against mature and immature stage lungworm. Antibiotics are also given to prevent secondary bacterial infection [21,34].
Control and prevention: Control of endoparasites is utmost desirable although internal parasite problem is usually related to management practices that increases exposure. Whereas ongoing preventive management practices minimizes losses caused by parasitic infection [25].
Control of lungworm infection in sheep may be achieved by pasture management. Animal must be removed from infected ground, placed on dry pasture and supplied with clean drinking water. Draining and resting pasture during dry summer kill many larvae that readily survive cold winter. Their feces should not be used for fertilizing lands on which crops for green feeding are grown, moist grasses should not be given to animals, and adult should not graze together with young stock [21,23].
Artificial immunization has been markedly successful using X- irradiated infective larvae and a commercial vaccine is now available. This vaccine which consists of two doses of 1000 irradiated larvae given at an interval of month has been used in hundreds of thousands animal in varies countries successfully [21,25].
Extermination of the intermediate (snail) host is additional measure for the control of metastrongylidae. This technique enables to control the nude slugs easily than shelled and spreading of lime has been recommended for this purpose [21,23].
Economic significance of lung worm infection: Lungworm infection causes production loss through direct consequences of clinical and sub- clinical infections resulting in low productivity due to stunted growth, reduced weight gain, poor feed utilization, conversion and lowered resistance or loss due to mortality or indirect loss associated with cost of treatment and control measures [35,36].
In Ethiopia, a rough estimation of economic loss due to decreased production found to be over 300 million Birr per year and the national value of their direct loss estimated to be of 550 million Ethiopian Birr [6].
Prevalence of lung worm infection in Ethiopia: Pulmonary verminous pneumonia due to various lungworm species has been reported to exist in Ethiopian sheep in different areas of the country. Some of existing reports on the prevalence of lungworm infection at different parts of the country are presented on the given (Table 2).
Materials and Methods
Study area
The study was conducted in Dangla woreda of Awi zone in Amhara region, Ethiopia. The study area is located in the Northwestern parts of Ethiopia, in Amhara region at Dangla town which is located at 78 kms away from Bahir Dar and 485kms away from Addis Ababa at 11.2670 North latitude and 36.8330 East longitude. The altitude of the area ranges from 1809-2137 meters above sea level. The mean annual minimum and maximum temperatures are 14 and 31°C respectively, with a minimum and maximum annual rainfall of 1500-2200mm. Generally, the climate of the area is characterized by woyna dega climatic condition. In the area, there are four main seasons in a year. Namely, the dry season winter (“Bega”) from December to February, spring (“Tsedey”) from March to May, summer (“Kremt”) from June to Augest and autumn (“Meher”) from September to November. The farming system in the area is mixed farming and sheep are the dominant animal species kept by farmers. The grazing land comprises waterlogged areas, forest margins, mountainsides, stony land and roadsides [37].
Study population
The study population included in this study were sheep having different sex, body condition score and age category and kept under extensive and semi extensive management system.
Study design and sampling method
Cross-sectional type of study was conducted from November 2014 to April 2015 to determine the prevalence of lungworm infection in sheep and associated risk factors in the study. For selecting PAs within the district, purposive sampling was employed for logistic reasons. Simple random sampling technique was the principal sampling method followed for sampling household and individual sheep for the study. The sample size desired for this study was determined by using the formula given by [38].
Where:
n=required sample size
Pexp=expected prevalence
d=desired absolute precision
1.962=z-value for the 95% confidence level
Accordingly the sample size was
Study methodology
384 fecal samples were taken from randomly selected sheep. Fecal samples from the selected animals were collected directly from the rectum by two fingers after wearing disposable gloves in a universal bottle and then transported to Dangla veterinary clinic as soon as possible and each sample was processed by modified Bearmann technique [39]. All samples were clearly labeled with the date of sampling, sex, age and body condition score. Age of animal was gathered from the owners and dentations.
The Laboratory work was done using Barman technique. 25 grams of fresh feces was weighed from each sample. The larvae and enclosed gauze fixed on to astringe rode were submersed in a clean glass tube which was filed with warm water left for 24 hours and the sediments were transferred to Petri dish for examination of L1 under lower power of microscope after siphoning off the supernatant. a drop of 1% iodine solution was added to the slide to immobilize the larvae were examined under microscope to identify the species of the larvae by morphological features of the larvae. Those not identified under microscopes, the examined samples were registered as negative for lungworm infection. In both cases, the result that was obtained for each sample was recorded to their corresponding specific animals [32,40,41].
Data analysis
The data was entered and managed in MS-Excel. All the data analysis was done by Statistical Package for Social Science (SPSS) soft ware version 20. Descriptive statistics such as percentages and frequency distributions were used to describe the nature and the characteristics of data. The prevalence of lungworm infection was analyzed using percentages. The association of different risk factors with the disease were computed by using Chi-square (x2) test.
Results
Of the total 384 sheep examined, 57 (14.8%) were found to be infected by one or more of the lungworm species. The lungworm species encountered during the study period were Dictyocaulus filaria, Muellerius capillaries and Protostrongylus rufescens as single and mixed infections with a prevalence of 24(6.3%), 15(3.9%), 11(2.9%) and 7(1.8%) respectively.
The sex of animals did not show statistically significant association with lungworm infection (x2 =0.112, p= 0.738). Age wise lungworm infection rate was higher in adult as compared to other age groups, but it was not statistically significant (P>0.05) (Tables 3-5).
Discussion
The overall prevalence of lungworm infection in sheep in the present study, 14.8% is in agreement (the same or nearly the same) with the works of [42], 15.07%, at Dessie and Kombolcha districts, Ibrahim and Degefa [43], 13.4% at Mekelle town and Yifat [16], 17.5% in and around Bahir-Dar town. The overall prevalence rate (14.8%) of the present study was considerably lower than the works of the following authors. These are Serkalem, et al., [44], 60.8% at Dale farm and abattoir, Yitagele, et al., [45] 46 and 56.3% by coproscopic and postmortem examinations respectively in North Gondar zone, Basaznew, et al., [46], 43.33% in Dessie Zuria District, Mekonnen, et al., [47], 33.83 and 32.6% based on coproscopic and postmortem examination respectively around Gondar and Tewodrose, et al., [48], 25.24% in and around Jimma town and. The grounds of low prevalence in this study could be attributed to the development of open-air clinic, careful management and increasing awareness of farmers to deworm their sheep against parasitic infections in the study area apart from geographical variations.
But the result of the present work was higher than the observations of other works; Frewengel [49], at Mekele town in different restaurants who reported 11.24%, Sisay [14], in Bahir Dar abattoir with a value of 13% and Ibrahim and Degefa [43], at Mekelle town who reported 13.4%. The differences in the prevalence of lungworm of sheep between this study and the above studies might be associated with nutritional status, level of immunity, method used for the detection of the larvae, management and regular deworming practices of the animal, rainfall, humidity, temperature and altitude differences, or difference in the study areas of topography, which has conducive environment for the survival of larvae and intermediate hosts, slug or snails which can influence the larvae in the respective study areas (http://www.esgpip.org) [50].
In the present finding both sexes showed equal susceptibility (they have equal chance of infection to the disease when they are allowed to graze at the same pasture) to infection with lungworms, hence sex dependent variation was not encountered. Alemu, et al., [10] and Dawit and Abdu [51], have also reported the same finding but Craig [52], Alemu, et al., [7] and Mihretab [53], have reported different result.
The lungworm infection prevalence was found to be significantly associated with the body condition of the study animal (X2 =15.483, p =0.001). A higher infection rate was observed in animals having poor body condition as compared to other body condition groups. Statistically significant difference was also observed in the infection rate among poor (26.3%), medium (13.6%) and good (7.1%) body condition in both single and mixed species infection. This is because of heavy parasitic loads, stress due to infections, disease which can cause sheep to lose conditions because they are not eating or the nutrients they eat are being diverted to parasites (http://www.esgpip.org).
The findings of the present study was in line with Mihretab [53], who reported that the prevalence was statistically significantly higher in animals with poor body conditions than medium or good body conditions in her survey. The achievable explanation for this observation could be due to immune-suppression in sheep with poor and medium body conditions, concurrent infection by other parasites including GIT helminthes and/or malnutrition [50]. Poorly nourished sheep appear to be less competent in getting rid of lungworm infection. Evidently, the infection with a parasite by itself might result in progressive emaciation of the animals [24]. Well nourishment and watering of sheep lead to less risk of helminthes infection as reported by Anne, et al., [32].
Regarding age, higher prevalence of lungworm infection was observed in the groups of adult (16.4%) as compared to age groups of young (15.6%) and old (13.6%). The difference was not statistically significant (p > 0.05). This might be associated with the frequent grazing behavior of adult animals those graze continuously following weaning from suckling of their dam by which natural immunity obtained from their mother becomes reduced and the appropriate environmental climatic conditions can also contribute to higher rate of infection when sheep are sold to or bought from different agro-climatic conditions (Dar, 2012). This finding is however disagree with Muluken and Tigist [54,55], findings in North and South Gondar zones and Tewodros, (2012) in and around Bahir Dar that they have founded the prevalence of lungworm infection is higher in ages of younger sheep than those of other age groups.
Three major important respiratory nematodes were identified by coproscopic examination of the sheep in the area. Dictyocaulus filaria was the most predominant lungworm species with a prevalence rate of 6.3% followed by M. capillaris 3.9%, P. rufescens 2.9% and mixed infections with two or three of the species, which accounted 1.8% and finally, the least prevalence was P. rufescens. This finding was in line with the studies of Nemat and Moghadam [56], in Tabriz, Dawit [51], around Jimma and Nuraddis and Yared [57], in Mekele.
In contrast to this finding [14], conducted research in [58], in Addis Ababa reported that M. capilaris was the most prevalent species. The possible explanation for the predominance of D. filaria in the study area might be attributed to the difference in the life cycles of the parasites. Thus, D. filaria has a direct life cycle and requires shorter time to develop to an infective stage. After ingestion, the larvae of these parasites can be shed with feces within 5 weeks [23]. Unlike to D. filaria, the transmission of P. rufescens and M. capillaris is epidemiologically complex event involving host, parasite and intermediate host. Because, P. refescens has indirect life cycle that requires longer time and wet or rainy warmer season to complete their complex life cycle in the presence of suitable intermediate hosts that create favorable condition for sporadic distribution it stands to the least prevalent rank. On the other hand, the low prevalence rate of M. capilaris and P. rufescens in the study area might be contributed to the fact that the study was done at the bigining of Autumn to the end of winter (November-March) which was short and erratic rainy and dry season) which does not favours the development of the snail intermediate hosts [59-61].
Conclusion and Recommendations
The result of the present study indicated that lungworm is one of helminthosis of sheep in the study area. Higher prevalence rate of lungworm infection was observed in animals with poor body condition. The prevalence of lungworm infection was higher in those sheep with poor body conditions than in those with medium and good body conditions. The prevalence of infection in adult animals is higher than other age groups and D. flaria is the dominant lungworm species in the study area. It can also be concluded that the infections caused by lungworms are significantly common in the study area and are important health problems of sheep which is speculated to cause heavy economic loss. Therefore, in line with the above conclusion the following recommendations have been forwarded:
Φ Due emphasis should be given for the prevention and control of lungworm infections.
Φ Further, detailed, seasonal and other risk factors study should be done to enable the development of appropriate control strategy.
Φ Education and awareness creation of farmers about lungworm.
Φ Treat sheep with broad-spectrum anthelmintic at the beginning of rain season would appear to be most effective.
Φ Farmers who keep sheep should be advised not to keep their animals in extensive management system of production and to give more emphasis to adult and poor body conditioned animals.
- ILCA International Livestock Center for Africa (1990) Annual Report, 1989 International Livestock Center for Africa (ILCA), Addis Ababa, Ethiopia 37-39.
- FAO, Food and Agricultural Organization (1986) Small ruminant production in the developing countries. In: proceedings of an Expert consultation held in Sofia, Bulgaria 8-12. Link: http://bit.ly/33kaFu2
- Wilsmore T (2006) Disease of small ruminants in Ethiopia, the veterinary epidemiology and economics research unit of agriculture’s policy and development the university of read, UK 602.
- Benin S, Ehui S, Pender J (2002) Policies for livestock development in Ethiopian high lands. Socio-economic and Policy Research Working Paper, Addis Ababa, Ethiopia 41. Link: http://bit.ly/2pOFrxB
- CSA, Central Statistical Authority (2009) Federal Democratic Republic of Ethiopia, Central Statistical Authority (CSA), Agricultural Sample Survey 2008/2009 (2001E.C). Report on Livestock and Livestock Characteristics, Addis Ababa 120.
- FAO, Food and Agricultural Organization (2009) Food and Agricultural Organizations of the United States (FAOSTAT) data.
- Alemu Y, Merkel R (2008) Sheep and Goat production Hand Book for Ethiopia, Ethiopian sheep and Goat productivity improvement program (ESGPIP) 2-6. Link: http://bit.ly/2QOliTu
- Sissay M (2007) Helminthes parasites of sheep and goats in eastern Ethiopia, EPID, and Antihelmintic Resistance and its management, Faculty of Veterinary Medicine and Animal Science, department of biological science and veterinary public health, division of parasitological and virology, Uppsala, Sweden 11-13. Link: http://bit.ly/34j4cB1
- EARO, Ethiopian Agricultural Research Organization (2000) Small Ruminant research strategy. Animal Science Research Directorate, Addis Ababa.
- Alemu S, Leykun E, Ayelet G, Zeleke A (2006) Study on small ruminants lung worms in northeastern Ethiopia. Vet Parasitol 142: 330-335. Link: http://bit.ly/33dzHeh
- Chauhan R, Agarwal D (2006) Text book of vet clinical and laboratory diagnosis. 2nd ed., Brothers Medical Publisher, New Delhi 81.
- Gelagay A, Leakemariam Y, Esayas G, Selam T, Kassahun A (2005) The Eth Vet J 9: 75-76.
- Radostitis O, Gay C, Blood D, Hinchclift K (2005) Disease associated with helminthes parasites in veterinary medicine: A text book of the disease of Cattle, Sheep, Pigs, Goats and Horse. 9th ed. Harcourt Publishers, Ltd. London 1564-1569.
- Sisay A (1996) Preliminary study on the prevalence of ovine lungworm infection in and around Bahir Dar, DVM thesis, Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia.
- Kassa B, Abdu K (2012) Standard Veterinary Laboratory in Debre tabor Awraja, DVM thesis, Faculty of Diagnostic Manual. Examination Techniques for small ruminants.
- Yifat D, Adugnaw M, Rahmeto A, Desie S (2013) Prevalence of Lungworm Infection in Sheep Around Bahir-Dar Town, Northern Ethiopia. Hawassa University, Hawassa, Ethiopia. Acta Parasitol Glob 4: 54-58. Link: http://bit.ly/2Ok2Pwb
- Jubb K, Kennedy P, Palmer N (2007) Pathology of domestic animal. 5th ed., Elsevier, New Delhi 2: 627-628. Link: http://bit.ly/2qBFvkL
- Yildiz K (2006) Prevalence of lungworm infection in Sheep and Cattle in the Kirikkale province. T Parazitol Derg 30: 190-193. Link: http://bit.ly/33j9k6R
- Urquhart G, Armour J, Duncan A, Dunnand F (1995) Jennings. Veterinary Parasitology. 1st ed., Black Well Publishing, London 270-290.
- Love S (2008) Sheep worm control and drench resistance. Agnote DAI/87 2nd ed., 25-66.
- Taylor M, Coop R, Wall R (2007) veterinary parisitology 3rd ed., Black Wall publishing, IOWA 94-97.
- Soulsby E (1986) Helminthes, arthropods and protozoa of domesticated animals. 7th ed., Bailliere Tindalla, London; 809. Link: http://bit.ly/2OgMb0r
- Soulsby E (1982) Helminthes, arthropods and protozoa of domestic animals.7th ed., Lea and Fediger, Philadelphia; 262-267. Link: http://bit.ly/2DekEGU
- Radostits O, Gay C, Blood D, Hinchclift K (2007) Veterinary medicine text book of disease of cattle, sheep, pigs, goats and horses, 9th ed., Elsevier Ltd, china 784-832.
- Upadahayay A (2005) Text book of preventive veterinary medicine as per VCL syllables. Inter book distri Co India 87-390. Link: http://bit.ly/34gH4mt
- Mandal S (2006) Veterinary parisitolgy at glass. 1st ed., International book distribution Co., Charbagh, Lucknow, India; 160-165.
- Hansen J, Perry B (1994) The epidemiology, diagnosis and control of Helminth parasites of ruminants, ILRA Nairobi Kenya 3-77. Link: http://bit.ly/37D7twT
- www.google.com.et
- Jubb K, Kennedy P, Palmer N (1993) Pathology of domestic animal, 4th ed., Elsevier, New Delhi 2: 520-521.
- Blood O, Radostitis O, Gray C (1989) Veterinary medicine a Text book of disease of cattle, sheep, pig, goat and horse. 7th ed., 1039-1044.
- Tizard I (1992) Introduction to Veterinary immunology 4th ed., Sun Co Philadelphia 263.
- Zajac A, Conboy G (2006) Veterinary clinical parisitolgy. 7th ed., Black Wall publishing, State Avenue 95.
- Anne M, Zajac D Gray A (2006) Veterinary Clinical Parasitology, 7th ed., Australia Blackwell publishing 11-14.
- DACA, Drug Administration Control Authority of Ethiopia (2006) Standard Treatment Guidelines for Veterinary Practice. 1st ed. , chamber printing house, Ethiopia 77. Link: http://bit.ly/34d7ZQd
- Ayalew A, Tewodros D, Alemayehu W (2011) Prevalence and risk factors of intestinal parasites among Dergi school children, North Gondar, Ethiopia. J Parasitol Vect Biol 3: 75-81. Link: http://bit.ly/34j9sof
- Desalegn L (1999) Proceeding of the 13th conference of Ethiopian Veterinary Association, development scheme, Report of a mission to Ethiopia; May 27- June 28; Working paper 23; Rome, Italy 1043-1044.
- (NMSA) National Meteorology Service Agency (2013).
- Thrusfield M (2005) Veterinary Epidemiology. 2nd ed., University of Edinburgh, Blackwell Sci 117-198. Link: http://bit.ly/2OFXOgC
- Charles M, Robinson E (2006) Diagnostic veterinary parasitology for veterinary technicians, 3rded., Mosby Inc. St. Louis, Missouri 243. Link: http://bit.ly/37z9Jp4
- Fraser C (1991) The Merck veterinary manual. A hand book of diagnosis, therapy and disease prevention and control for the veterinarians. 9th ed. Mark and co. Inc, Rahway, Nit, USA; 714-814. Link: http://bit.ly/2KPkSIH
- Urquhart G, Armour J, Duncan J, Dunn A, Jennings F (1994) Veterinary parisitolgy 2nd ed. , Long man Eng. lang. soci ., Blackwell Publishing, USA 39-58.
- Teffera S (1993) Prevalence of Ovine Lungworms Protozoa around Dessie and Kombolcha, DVM thesis, Addis Ababa University, Debrezeit, Ethiopia.
- Ibrahim N, Yifat D (2012) Prevalence of Ovine with Lung Worm Infection in Mekelle Town, North Ethiopia. Int J Vet Med 9: 1-15.
- Serkalem K, Sissay M, Mulugeta D (2014) On farm and Abattoir study of Lungworm infection of small ruminants in selected areas of Dale District, Southern Ethiopia. Int J Curr Microbiol App Sci 3: 1139-1152. Link: http://bit.ly/35t0uoz
- Yitagele T, Ketema T, Getasew F, Nigatu K (2013) Prevalence of lungworm infection in small ruminants in North Gondar zone, Faculty of Veterinary Medicine, Haramaya University, Ethiopia. J Parasitol Vector Biol 5: 40-45. Link: http://bit.ly/2DcoWhZ
- Basaznew B, Ayalew, Achenef M (2012) Ovine Lungworm Infection: Prevalence, Species Composition and Associated Risk Factors in Dessie Zuria District, Northeastern Ethiopia. Afric J Basic App Scien 4: 73-76. Link: http://bit.ly/2OKHhry
- Mekonnen A, Abebe F, Yohannes E (2011) Study on the Prevalence of Lungworm Infection in Small Ruminants in Gondar Town, Ethiopia. Vet Res 10: 1683-1687. Link: http://bit.ly/2XQDPQw
- Tewodros F, Yeshiwas S, Mersha C, Nibret M (2012) Prevalence of Lungworm Infection in Small Ruminants in and Around Jimma Town, Southwest Ethiopia. Faculty of Veterinary Medicine, University of Gondar, Gondar. Eth Glob Vet 9: 580-585. Link: http://bit.ly/2OA7aKw
- Frewengel S (1995) Prevalence of Ovine Dictyocaulosis in and around Mekele (Tigray), DVM thesis, Faculty of Veterinary Medicine, Addis Ababa University, Debre Zeit, Ethiopia.
- Thomson E, Orita G (1988) Seasonal prevalence of Protostrongylid and Dictyocaulus species of lungworms in sheep in Western Syria. Trop Anim Hlth Prod 20: 187-189. Link: http://bit.ly/34i8Zmd
- Dawit W, Abdu M (2012) Prevalence of Small Ruminant Lung Worm Infection in Jimma Town. Glob Vet 8: 153-159. Link: http://bit.ly/2QYpDnd
- Craig T (1998) Epidemiology of internal parasites, effect of climate and host on reproductive cycle in parasite survival. Small ruminants for the mixed animal practitioner, Las Vagas, Blackwell publishing 11-14.
- Mihreteab B, Aman A (2011) Ovine Lungworm in Tiyo District, South-East Ethiopia: Prevalence, Effect of Altitude and Host Related Risk Factors. Glob Vet 7: 219-225. Link: http://bit.ly/34gCLaN
- Muluken B (2009) Preliminary study on presence of ovine lungworm infection in around Bahir Dar. DVM thesis, Facullity of Veterinary Medicine, Jimma University, Jimma, Ethiopia.
- Tigest B (2009) Study on prevalence of lungworms in north and southern Gondar. DVM. Thesis, University of Gondar, Faculty of Veterinary Medicine, Gondar, Ethiopia.
- Nemat E Moghadam G (2010) Survey on Annual Infestation of Sheep with Lung Worms based on Fecal Test and Slaughter House Study in Tabriz. J Vet Res 64: 339-342. Link: http://bit.ly/2OTXOK7
- Nuraddis I, Yared D (2012) Prevalence of Ovine Lung Worm Infection in Mekelle Town, Ethiopia. Int J Vet Med 9: 25-27.
- Mezgebu M (1995) A survey on ovine lungworm infection in Addis Ababa. DVM thesis. Addis Ababa University, Faculty of Veterinary Medicine, Debre-Zeit, Ethiopia.
- Kahn C (2005) The Merck Veterinary Manual, 9th ed. Merck and white house station 215-256. Link: http://bit.ly/34gCchb
- ESGPIP (2000) Body condition scoring of sheep and goat in Ethiopia. ESGPIP Tech Bull 8.
- ESGPIP (2000) Estimation of weight and age of sheep and goats in Ethiopia. ESGPIP Tech Bull 23.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
