International Journal of Veterinary Science and Research
Mesenchymal stem cells from the domestic ungulates: trends and outliers
Anna A Tvorogova1,2, Anastasia V Kovaleva1,2 and Aleena A Saidova1,2*
2Center of Experimental Embryology and Reproductive Biotechnology, 127422, Moscow, Russia
Cite this as
Tvorogova AA, Kovaleva AV, Saidova AA (2018) Mesenchymal stem cells from the domestic ungulates: trends and outliers. Int J Vet Sci Res 4(1): 023-031. DOI: 10.17352/ijvsr.000032Mesenchymal stem cells (MSCs) are a valuable source for regenerative therapy and tissue engineering. MSCs are multipotent adherent stem cells that can be isolated from different adult and fetal tissues. In contrast to human MSCs, MSCs from large animal models have not yet been described by the uniform criteria, which include the characteristic phenotype of surface molecules, expression of stemness markers and differentiation potencies. The current review describes state of the art for characterization for MSCs from three species of domestic ungulates, including cattle (Bos taurus), swine (Sus scrofa) and sheep (Ovis aries). The comparative analysis of surface phenotype, gene expression and differentiation capacities of MSCs from different origins allows defining the consensus phenotype of bovine, ovine and porcine MSCs. We also discuss the major data discrepancies and pitfalls that are complicating the successful research of MScs from domestic livestock. This review emphasizes the pressing need for the unification of mesenchymal stem cell criteria in the veterinary field.
Introduction
Stem cells are a specific group of cells that have two significant hallmarks, which are the self-renewing capacity and the capability to differentiate into various adult cell lines. Stem cells can be divided into embryonic stem cells (ESCs) and adult stem cells, depending on the developmental stage of the tissue source [1]. In contrast to the totipotent zygote and multipotent ESCs from the inner mass of the blastocyst, adult stem cells are termed multipotent, as they can differentiate into cell types of only one germ layer of their origin [2]. Some multipotent stem cells seem to have more plasticity, demonstrating the ability to multi-lineage differentiation, though this property is almost never confirmed in studies in vivo studies and no functional analysis of such cells is usually performed [3,4].
Mesenchymal stem cells (MSCs), which were firstly isolated from the bone marrow of the guinea pig by A. Friedenstein [5], represent the adult stem cells with a spindle-like morphology that can adhere to plastic and share a common immunophenotype CD29+/CD44+/CD73+/CD105+/CD106+/CD166+ and CD11-/CD14-/CD34-/CD45-/CD19-[6]. Since most of these molecules are expressed on different cells from the hematopoietic lineage and endothelium, a full and comprehensive panel of surface antigens must be used in any MSC study for unequivocal detection of MSCs.
Expression of constitutive MSC markers can be additionally changed due to various environmental factors, like infection, media conditions an hormones. Claessen et al. [7], showed that equid herpesvirus infection (EhV) led to the significant downregulation of CD29, CD105 and MHC1, while expression of CD44 and CD90 remained unchanged. Moreover, steroid hormones influenced the expression levels of CD29, CD44, CD73, CD90, CD105 in MSCs from the human endometrium. Interestingly, photon and carbon ion radiation did not change the expression of CD29, CD44, CD73, CD90 and CD105 on mRNA level [8].
The primary source of MSCs is bone marrow. However, they can also be isolated from the adipose tissue, heart, dermis and fetal organs and fluids [9]. Relatively simple isolation protocols, accessibility and multipotential capacities of MSCs together with limited ethical concerns implemented the growing body of knowledge of these cells both in the fundamental and practical field. Several groups reported the use of MSCs for the regenerative therapy of heart, bone, cartilage, spinal cord and skin defects and traumas on human and animal models [10-13]. In contrast to human MSC research [6], no uniform criteria of MSC are available for veterinary models in general and domestic ungulates in specific.
Domestic ungulates represent the superorder Ungulata, which is a diverse group of primarily large mammals. Three species of domestic ungulates, cattle (Bos taurus), sheep (Ovis aries) and pig (Sus scrofa) have outstanding economic importance in the livestock industry, being a source of nutrition, leather, and wool [14]. Despite the manifested significance of these animals and their relevance as large animal experimental models, the data body on the MSCs from the domestic ungulates is incomparable to the data for human and rodent MSCs. The main problems of MSC research field is the limited availability of species-specific or cross-linking antibodies for the veterinary study [15], the lack of clarification for MSC definition and nomenclature [16] and apparent differences from human and rodent MSCs in culturing conditions and expression of stemness markers, that makes the established protocols for primates and rodents inapplicable for the veterinary field and large animal models.
The purpose of this review was to summarize the currently available information on isolation, characterization, conventional markers and differentiation capacities of three species of domestic ungulates, with emphasis on the discrepancies in phenotype and molecular markers for MCSs from different tissue sources. Our ultimate goal was the accurate and comprehensive comparison of the research results from different groups to refine and to uniform the criteria of MCSs from domestic ungulates.
Cattle (Bos taurus)
Unlike human, rodent and even swine MSCs, bovine MSCs (bMSCs) are still underexplored. The list of the described surface molecules of bMSCs from different tissue sources is presented in table 1. Most of them represent the superfamily of cell adhesion molecules (CD44, CD106, CD146, CD166) or integrins (CD11a, CD29, CD49d), while the function of others (CD90, CD105) has not yet been fully elucidated. Regardless the tissue source, all bMSCs are positive for CD29, CD44, CD73, CD90, CD105 and CD166 and negative for CD9, CD11a, CD14, CD79 and CD45. In most studies, bMSCs are also negative for CD34, except the study by Rossi et al. [17], who demonstrated the high expression of CD34 in a subpopulation of bMSCs from the amniotic fluid at different trimesters of pregnancy. At the same time, Chang et al. [18], showed the absence of CD34 expression in bMSCs from the amniotic fluid taken at the first trimester of pregnancy. The other discrepancy concerns the expression of CD271. Similar to the studies on human MSCs (reviewed at Rojewski et al. [19]), a single study [20], reports a small population of bMSCs from the bone marrow that expresses CD271, while there is no evidence on CD271 positive cells in all other bovine tissues. The other problem with the detection of protein expression in bovine cells is the absence of bovine-specific antibodies and the use of human cross-linking antibodies for detection of bovine antigens. In some cases, it may lead to the false results, like in study by Rossi et al. [17], where CD73 was not detected on bMSC from the amniotic fluid in contrast to other studies [18,21]. Another critical issue concerns the change of surface antigens expression through the passaging that was demonstrated for CD90 and CD44 [17] and also reported for human MSC [22]. It is noteworthy that in some cases the presence or absence of expression for several surface molecules was confirmed only on mRNA level, and that a confirmation of expression on protein level with large-scaled multicolor flow cytometry of bMSC from different tissue sources is still a problem to be solved. One more significant challenge for future investigations is the morphological similarities of bMSC and fibroblasts, which exhibit spindle-like morphology and can adhere to plastic [23]. Moreover, fibroblasts express some surface molecules of MSCs that has been extensively reviewed at Ichim et al. [24], Chang et al. [25], demonstrated the multilaneage differentiation of fibroblasts from various tissues, that again raises the question about the proper detection, isolation, and determination of bMSCs and drives the need of more complex and comprehensive investigations of the bMSC phenotype.
Apart from the phenotype of the surface markers, the molecular signature of mesenchymal cells often includes a typical pattern of the pluripotency markers like Oct4, Sox2, Rex1, Nanog and SSEA family proteins. Although bMSCs are multipotent, not pluripotent stem cells, most of these markers, from the analogy with hMSCs [9], are widely used to confirm the stemness properties. bMSCs from the bone marrow express Nanog and Oct4 both on mRNA and protein level, while the results for bMSCs from other tissues are very controversial (Table 2). The expression of Oct4, Naanog, and Sox2 in bMSCs from the amniotic fluid id very controversial [17,21,26], however, the same has been shown for hMSCs [27] and the discrepancy in the results may occur to the heterogeneity of bMSC population and detection methods. The data for bMSCs from the adipose tissue and umbilical cord (Warton’s jelly) are not comparable, and the molecular signature of bMSCs from these sources is still unclear.
Moreover, the levels of mRNA pluripotency markers can change unpredictably during the passaging and even under differentiation conditions. Rossi et al. [17], showed that the mRNA expression of NANOG decreased during chondrogenic differentiation of bone marrow-derived bMSCs, but increased during osteogenic differentiation and did not change in adipocytes. Thus, the presence of stemness markers in bMSCs from different sources needs the comprehensive revision with simultaneous detection of these molecules both on mRNA and protein level.
The next part of bMSCs characteristics is the demonstration of multi-lineage differentiation under specific conditions. Using the commercially available induction media, bMSCs from the bone marrow, umbilical cord, adipose tissue, and amniotic fluid can differentiate towards chondrocytes, adipocytes, and osteocytes (Table 3), while their differentiation towards other lineages is less straightforward. In one study bMSCs from the bone marrow differentiated into hepatocytes, and in three studies bMSCs form the bone marrow, amniotic fluid, and umbilical cord demonstrated the neurogenic potential [21,28,29]. It means that bMSCs have the trans-differentiation potential, as the neurons belong to the ectoderm, hepatocytes have the endodermic origin, while all other cells have the mesodermal origin. Given the expression of the ESC cells markers and differentiation capacities, bMSCs retain the higher level of cell plasticity than it is commonly believed.
Specific histological stainings are used to confirm the functional properties of the differentiating cells.
The most common histological techniques that are used for the description of bMSCs differentiation capacities are described in table 3. To identify calcium and phosphate deposits that appear in cells during osteogenesis, specific matrix stains like Alizarin Red and Von Kossa are both widely used for bMSCs and recommended in conventional MSC characterization protocols [6]. Alcian blue and Toluidine blue are applied to stain glycosaminoglycans during chondrogenic differentiation, while Oil Red that stains neutral lipid droplets is used to describe the adipogenesis of bMSCs.
Another approach to confirm the differentiation capacities of bMSCs is RT PCR evaluation of mRNA expression for genes that are characteristic for each particular cell type (Table 3). Common molecules that confirm the chondrogenic differentiation are Sox9, collagen type II and aggrecan. Aggrecan is cartilage-specific proteoglycan, a member of chondroitin sulfate proteoglycan family that plays several roles in the maintenance of cartilage tissue [30]. Sox9 is a transcription factor that plays a pivotal role in collagen formation and negatively regulates cartilage vascularization [31], and collagen type II is a major component of extracellular matrix in the cartilage tissue [32]. Essential markers of adipocytes include PPARγ, which is a specific transcription factor that affects fatty acids metabolism and activates adipocyte-specific genes like adiponectin and resistin [33], leptin (LEP), which is a hormone made predominantly by adipose cells and plays a critical role in homeostasis of the adipose tissue [34] and lipoprotein lipase (LPL) that controls entry of fatty acids into adipocytes [35]. Osteogenic differentiation for bMSCs is usually confirmed by the expression of osteopontin, osteocalcin, Runx2, and collagen type 1. Osteopontin is a glycoprotein that regulates biomineralization of bones [36], osteocalcin is osteoblast-specific hormone that regulates glucose homeostasis in bone tissue [37], Runx2 is an essential transcription factor for early osteogenesis, and collagen type I is also expressed by early fibroblasts, since it forms the primary network for further mineralization process [38]. Unlike the expression of stemness markers and immunophenotype, there are no obvious discrepancies in the set of differentiation markers and techniques applied to bMSCs. The only controversy that was found in the literature is an expression of collagen type I in a subset of a bMSCs from the amniotic fluid [17].
Pig (Sus scrofa)
Due to the similarities of the immune system and MHC similarities [39,40] porcine MCS (pMSCs) are much more explored compared to their bovine counterparts. Due to the higher similarity of surface epitopes for the majority of antigens, the expression for almost all surface molecules of for pMSCs is confirmed by flow cytometry and immunofluorescence (Table 4). The consensus phenotype of pMSCs regardless of the tissue source is CD29+/CD44+/CD90+/CD105+ and CD14-/CD34-/CD45-. Importantly, the positive expression of CD73, which was confirmed both on mRNA and protein level for bMSCs, was not included in common phenotype of pMSCs, and the only group that evaluated the expression of CD73 on pMSCs isolated from bone marrow reported the absence of cross-linking for this molecule with antihuman antibodies (the same has been shown for CD19 and CD79b) [39], and it is unclear, whether this marker was not detected in other studies due to the lack of expression or the absence of species-specific antibody. mRNA expression of CD73 was confirmed on mRNA level for pMSCs from adipose tissue [41].
An important consideration for cell-based therapy and translational research is the possibility of substitution of MSCs from the bone marrow with skin- or adipose tissue-derived MSCs. Ock et al. [42] were the first who compared phenotypic characteristics and functional properties of MCSs from different sources on a porcine model. pMCSs from bone marrow and skin had comparable levels of CD90 and were uniformly negative on CD45, but CD29 expression was substantially upregulated in pMSCs from bone marrow compared to skin-derived cells. Kang et al. [43], compared expression of surface molecules for pMSCs from the umbilical cord and bone marrow and showed that expression levels of CD29 and CD44 are similar in these cells, whereas CD90 is upregulated in MSCs from the umbilical cord compared to bone-marrow-derived cells, and bone marrow-derived cells demonstrate slightly increased expression of CD45 (6,02%) compared to the lack of CD45 expression for MSCs from the umbilical cord. Full and comprehensive comparison of phenotype profile between MCS from different tissue sources is hindered because of more complex derivation and culturing protocols for porcine MSCs and the accessibility of tissue material, as, for example, only a few studies describe the phenotype of the umbilical cord or amniotic fluid-derived porcine MSCs [43,44], compared to the bovine model. Another critical issue of the translational research and preclinical models is the comparison of the phenotype and functions of animal and human MSCs. Noort et al. [39] in a detailed investigation showed that human and porcine MCS share a common surface phenotype and proliferative capacities, and the differences in expression of surface markers between these cells can be attributed to the lack of cross-reactivity for the corresponding antibodies. Like for bovine MSCs [20], the selection of CD271-positive cells both for human and porcine MSCs can lead to a substantial increase in MSC frequency in the clonal CFU-forming assay [39]. The only described difference in porcine and human MSCs after clonal enrichment was the decreased expression of CD146 that might reflect the difference in the adhesive and migrational properties of these cells, but still is a subject for further investigation.
In contrast to the description of surface molecules and consensus phenotype, the set of stemness markers of pMSCs is described less thoroughly. The expression of Oct3/4, Nanog and Sox2 was confirmed on protein and mRNA level only for skin-derived pMSCs (Table 2) [45-47], while expression of the same markers for bone marrow-derived pMSCs was reported in the only study by Ock et al. [42] and the other group reported the absence of expression of SSEA1 and SSEA4 on the same cells by flow cytometry, so it is still unclear, whether these molecules are absent on pMSCs, like it was reported for rodent cell lines [48], or again this fact should be attributed to the lack of appropriate species-specific antibodies. To date, no information is available on the expression of stemness-associated molecules for adipose tissue-derived pMSCs and stem cells from fetal organs and fluids. However, in contrast to bMSCs, there are no significant discrepancies in the expression of Oct4/Sox2/Nanog between cells from different tissue sources and within one tissue type.
Like for bMSCs, multilineage potential of porcine MSCs has been confirmed for the basic in vitro tri-lineage differentiation into chondrocytes, adipocytes, and osteocytes (Table 3), that confirms the stemness of these cells according to the ISCT criteria [6]. Compared to bovine MSCs, which demonstrate spontaneous differentiation into chondrocytes in vitro system in the absence of the supplementary chemicals [49], MSCs from swine are more capable of differentiating through the adipogenic lineage. This differentiation is accompanied by high levels of lipid accumulation and expression of adipose-tissue related genes (PPARγ, aP2, perilipin, and LPL). Chondrogenic differentiation of porcine MSC is less abundant, and no specific studies were dedicated to the chondrocyte differentiation of porcine MSC in context of cartilage repair. Expression of cartilage-related genes under chondrogenic differentiation was described only for pMSCs from the skin and umbilical cord [43,44,46]. Multipotent mesenchymal stem cells of mesodermal origin should also be committed to the myogenic differentiation, but literature mining has revealed the only study that demonstrated the myogenesis of pMSCs from the bone marrow [50], where differentiated cells were morphologically similar to myocytes (three or more nuclei, muscle-tube structures) and expressed MyoD, Myf5, myogenin, and desmin (a myogenesis-specific markers) both on mRNA and protein level in a time-dependent manner. The ability of pMSCs from different origins to differentiate through the myogenic lineages has to be further elucidated.
An essential property of porcine MSCs is their high plasticity and trans-differentiation potential to ectodermal and endodermal lineages. pMSCs from different origins successfully differentiated into ectodermal neuron-like cells and expressed specific markers of neurons and glia including β III tubulin, MAP2, NeuN, NF-M and GFAP (Table 3). Importantly, pMSCs derived from the umbilical cord are more capable for neurogenic differentiation than bone marrow-derived stem cells and expressed higher levels of NGF (neuronal growth factor) and nest in that was confirmed through in vivo transplantation on a mouse model of Parkinson disease [43]. Studies on the neuronal regeneration of pMSCs from the adipose tissue and umbilical cord confirmed the equal potencies of these cells to the convenient bone marrow-derived MSCs that allows using these cells in translational research models and helps to avoid the highly invasive and painful procedures of bone marrow puncture.
In addition to the neurogenic ectodermal differentiation, porcine MSCs showed the potencies to endodermic lineage differentiation in vitro system. Recent studies confirmed the capacity of bone marrow and adipose tissue-derived pMSCs to differentiate into hepatocytes with sustained hepatocyte-specific functions, like urea and glycogen synthesis and cytochrome p450 expression [51]. It is noteworthy that differentiated hepatocytes had the characteristics similar to the primary hepatocytes from swine liver [51,52] that makes these cells applicable for translational research and regenerative therapy.
Sheep (Ovis aries)
Ovine MSCs (oMSCs) seem to be the most questionable type of ungulate stem cells due to many controversies in their phenotype (Table 5). These controversies are reported even for the common MSC markers, like CD29, CD90, and CD105. Caminal et al. [53] reported that 96.6% of the overall bone marrow-derived oMSCs express CD90, while Desantis et al. [54], revealed only 12% of CD90-positive oMSCs in bone marrow, and Rentsch et al. [55] demonstrated heterogeneity of CD90 expression in oMSCs with immunofluorescence method. Moreover, Adamzyk et al. [56] demonstrated the various levels of CD90 for cells in different passages and media conditions. The same is true for CD29, as only 20% of CD29 positive oMSCs in bone marrow were detected by Adamzyk et al. [56], whereas McCarty et al. demonstrated that almost 99% of oMSCs were positive for this marker [57], and Boos et al. [58] confirmed high level of CD29 expression by RT PCR. CD105 expression in oMSCs also depends on the composition of culture media [56], Caminal et al. [53] reported the presence of CD105 on 55.6% of oMSCs, while Rentsch et al. [55] described all oMSCs as strongly CD105-positive with immunofluorescence method. Heterogeneity of ovine MCSs was demonstrated not only within same tissue type but for MCSs from different origins. Weber et al. [59] compared phenotypes of ovine MSCs from bone marrow and amniotic fluid using immunofluorescence and flow cytometry methods and revealed the discrepancies of CD106 expression for these cells. Martinez-Lorenzo et al. [60] compared ovine MSCs to human MSCs from bone marrow and demonstrated the upregulation of CD31, CD54, CD106 and CD117 and downregulation of CD49d, CD71, CD73, and CD105 in ovine MSCs compared to their human counterparts. The same group reported the two-fold downregulation of CD106 and CD49f expression during the first passage, and that fact limits the comparison of oMSCs phenotype for cells taken on different passages. In a very comprehensive study by Sanjuro-Rodriguez et al. [61], it was emphasized that most of the anti-human and anti-rat antibodies (except for CD29 and CD166) show the absence of cross-reactivity to ovine antigens. Given all the limitations listed above, the consensus phenotype of ovine mesenchymal stem cell to date should be described as CD13+/CD29+/CD44+/CD90+/CD166+ and CD31-/CD34-/CD45-. However, in this case, the first step of the phenotype evaluation must be the obligate prerequisite test for antibodies cross-reactivity and confirmation of expression both on mRNA and protein level. General recommendations for antibodies testing are explicitly provided for equine MSCs [62] and can be applied for ovine MCSs.
Expression of stemness markers in ovine MSCs is described in a few studies and cannot be summarized for mesenchymal stem cells from different origins. oMSCs from bone marrow express SOX2 on mRNA level and are positive for SSEA4 on protein level [61], oMSCs from amniotic fluid express STAT3 and NANOG mRNA [59] and express OCT4 confirmed by immunofluorescence [63]. Full expression profile of stemness markers in ovine MSCs from different origins has to be further elucidated.
According to the literature data, ovine MSCs from bone marrow, umbilical cord, peripheral blood, and amniotic fluid are capable for classic tri-lineage adipogenic, osteogenic and chondrogenic differentiation in vitro assays (Table 3). Expression of lineage-specific markers of ovine MSCs during differentiation is similar to that for bMSCs and pMSCs. However, the general plasticity of ovine MSC, in contrast to other species, is limited to mesodermal lineage, and ectodermal or endodermal differentiation potencies are still not confirmed for these cells. Taken together, these data imply the pressing need for ovine MSC characterization both for in vitro and in vivo assays.
Conclusions and Future Perspectives
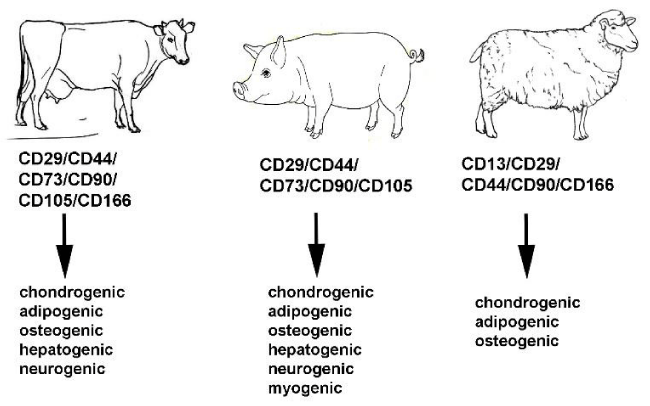
Mesenchymal stem cells of domestic ungulates can be isolated from different adult and fetal tissues, share some of the common phenotype molecules and are capable of tri-lineage mesodermal differentiation (Figure 1). However, immunophenotype of mesenchymal stem cells from swine, sheep, and cattle demonstrate apparent differences in expression of common and rare surface markers, these cells are heterogeneous on stemness markers and have different plasticity towards the trans-differentiation on ectodermic and endodermic lineages. The critical considerations for mesenchymal cells of domestic ungulates are the comparison of all molecules and stemness markers for cells from different origins, confirmation of expression of surface antigens and stemness markers on mRNA and protein level, and comprehensive analysis of surface phenotype and differentiation potencies depending on the culturing conditions and passaging time. All these considerations will allow the more comprehensive analysis to find uniform criteria of mesenchymal stem cells from the large animal models as for human mesenchymal stem cells and provide the data for the breakthrough in this innovative field.
The author has received no funding for this manuscript.
Author contributions
Anna V. Tvorogova and Anastasia V.Kovaleva drafted the manuscript, Aleena A. Saidova designed, drafted and critically revised the manuscript.
- Fortier LA (2005) Stem cells: classifications, controversies, and clinical applications. Veterinary surgery 34: 415-423. Link: https://tinyurl.com/y7mlmk7u
- Lakshmipathy U, Verfaillie C (2005) Stem cell plasticity. Blood reviews 191: 29-38. Link: https://tinyurl.com/y9op9hev
- Ishikawa T, Banas A, Hagiwara K, Iwaguro H, Ochiya T (2010) Stem cells for hepatic regeneration: the role of adipose tissue derived mesenchymal stem cells. Current stem cell research & therapy 5: 182-189. Link: https://tinyurl.com/y7wcl9zu
- Kopen GC, Prockop DJ, Phinney DG (1999) Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proceedings of the National Academy of Sciences of the United States of America 96: 10711-10716. Link: https://tinyurl.com/y7t5wgeq
- Friedenstein AJ, Chailakhjan RK, Lalykina KS (1970) The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell and tissue kinetics 3: 393-403. Link: https://tinyurl.com/yadxx7kf
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317. Link: https://tinyurl.com/y75huyra
- Claessen C, Favoreel H, Ma G, Osterrieder N, De Schauwer C,et al. (2015) R Equid herpesvirus 1 (EHV1) infection of equine mesenchymal stem cells induces a pUL56-dependent downregulation of select cell surface markers. Veterinary microbiology 176: 32-39. Link: https://tinyurl.com/ydcv2ulk
- Nicolay NH, Lopez Perez R, Saffrich R, Huber P (2015) E Radio-resistant mesenchymal stem cells: mechanisms of resistance and potential implications for the clinic. Oncotarget 6: 19366-1980. Link: https://tinyurl.com/ycqdhybh
- Riekstina U, Cakstina I, Parfejevs V, Hoogduijn M, Jankovskis G,et al. (2009) J Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem cell reviews 5: 378-386. Link: https://tinyurl.com/y6w9ths8
- Borjesson DL, Peroni JF (2011) The regenerative medicine laboratory: facilitating stem cell therapy for equine disease. Clinics in laboratory medicine 31: 109-123. Link: https://tinyurl.com/ybonutzq
- Meirelles Lda S, Fontes AM, Covas DT, Caplan A (2009) I Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine & growth factor reviews 20: 419-427. Link: https://tinyurl.com/yd4maf8p
- Peroni JF, Borjesson DL (2011) Anti-inflammatory and immunomodulatory activities of stem cells. The Veterinary clinics of North America. Equine practice 27: 351-362. Link: https://tinyurl.com/y7t75nag
- Lee KB, Hui JH, Song IC, Ardany L, Lee E (2007) H Injectable mesenchymal stem cell therapy for large cartilage defects--a porcine model. Stem cells (Dayton, Ohio) 25: 2964-2971. Link: https://tinyurl.com/y9s2q22s
- Thornton PK (2010) Livestock production: recent trends, future prospects. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 365: 2853-2867. Link: https://tinyurl.com/jpmsrpl
- Rozemuller H, Prins HJ, Naaijkens B, Staal J, Buhring HJ, et al. (2010) C Prospective isolation of mesenchymal stem cells from multiple mammalian species using cross-reacting anti-human monoclonal antibodies. Stem cells and development 19: 1911-1921 Link: https://tinyurl.com/ycwp5x3k
- Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, et al. (2005) A Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 7: 393-395. Link: https://tinyurl.com/y8jbmyv5
- Rossi B, Merlo B, Colleoni S, Iacono E, Tazzari PL, et al. (2014) Isolation and in vitro characterization of bovine amniotic fluid derived stem cells at different trimesters of pregnancy. Stem cell reviews 10: 712-724. Link: https://tinyurl.com/y927pdtc
- Chang LB, Peng SY, Chou CJ, Chen YJ, Shiu JS, et al. (2018) Therapeutic potential of amniotic fluid stem cells to treat bilateral ovarian dystrophy in dairy cows in a subtropical region. Reproduction in domestic animals = Zuchthygiene 53: 433-441. Link: https://tinyurl.com/create.php
- Rojewski MT, Weber BM, Schrezenmeier H (2008) Phenotypic Characterization of Mesenchymal Stem Cells from Various Tissues. Transfusion medicine and hemotherapy: offizielles Organ der Deutschen Gesellschaft fur Transfusionsmedizin und Immunhamatologie 35: 168-184. Link: https://tinyurl.com/y9mh39ra
- Jones EA, Crawford A, English A, Henshaw K, Mundy J, et al. (2008) Synovial fluid mesenchymal stem cells in health and early osteoarthritis: detection and functional evaluation at the single-cell level. Arthritis and rheumatism 58: 1731-1740. Link: https://tinyurl.com/ybfnnfhp
- Corradetti B, Meucci A, Bizzaro D, Cremonesi F, Lange Consiglio A (2013) Mesenchymal stem cells from amnion and amniotic fluid in the bovine. Reproduction (Cambridge, England) 145: 391-400. Link: https://tinyurl.com/yanudwf6
- Halfon S, Abramov N, Grinblat B, Ginis I (2011) Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem cells and development 20: 53-66. Link: https://tinyurl.com/ya63t2tz
- Alt E, Yan Y, Gehmert S, Song YH, Altman A, et al. (2011) Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biology of the cell 103: 197-208. Link: https://tinyurl.com/y9rjmfkh
- Ichim TE, O'Heeron P, Kesari S (2018) Fibroblasts as a practical alternative to mesenchymal stem cells. Journal of translational medicine 16: 212. Link: https://tinyurl.com/ybljcd9c
- Chang Y, Guo K, Li Q, Li C, Guo Z, et al. (2016) Multiple Directional Differentiation Difference of Neonatal Rat Fibroblasts from Six Organs. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology 39: 157-171. Link: https://tinyurl.com/y9tz7fro
- Gao Y, Zhu Z, Zhao Y, Hua J, Ma Y, et al. (2014) Multilineage potential research of bovine amniotic fluid mesenchymal stem cells. International journal of molecular sciences 15: 3698-3710. Link: https://tinyurl.com/ybo945by
- Zia S, Toelen J, Mori da Cunha M, Dekoninck P, de Coppi P, et al. (2013) Routine clonal expansion of mesenchymal stem cells derived from amniotic fluid for perinatal applications. Prenatal diagnosis 33: 921-928. Link: https://tinyurl.com/y9hexndl
- Duenas F, Becerra V, Cortes Y, Vidal S, Saenz L, et al. (2014) A Hepatogenic and neurogenic differentiation of bone marrow mesenchymal stem cells from abattoir-derived bovine fetuses. BMC veterinary research 10: 154. Link: https://tinyurl.com/y9hexndl
- Lange-ConsiglioA, Perrini C, Bertero A, Esposti P, Cremonesi F, et al. (2017) Isolation, molecular characterization, and in vitro differentiation of bovine Wharton jelly-derived multipotent mesenchymal cells. Theriogenology 89: 338-347. Link: https://tinyurl.com/yauas72a
- Watanabe H, Yamada Y, Kimata K (1998) Roles of aggrecan, a large chondroitin sulfate proteoglycan, in cartilage structure and function. Journal of biochemistry 124: 687-693. Link: https://tinyurl.com/ycmz6cm5
- Hattori T, Muller C, Gebhard S, Bauer E, Pausch F, et al. (2010) SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development (Cambridge, England) 137: 901-911. Link: https://tinyurl.com/y83jmzde
- Eyre D (2002) Collagen of articular cartilage. Arthritis research 4: 30-35. Link: https://tinyurl.com/yd7lf7u4
- Sharma AM, Staels B (2007) Review: Peroxisome proliferator-activated receptor gamma and adipose tissue--understanding obesity-related changes in regulation of lipid and glucose metabolism. The Journal of clinical endocrinology and metabolism 92: 386-395. Link: https://tinyurl.com/ycpn2ry7
- Stern JH, Rutkowski JM, Scherer PE (2016) Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk. Cell metabolism 23: 770-784. Link: https://tinyurl.com/y927fctn
- Weinstock PH, Levak-Frank S, Hudgins LC, Radner H, Friedman JM, et al. (1997) L Lipoprotein lipase controls fatty acid entry into adipose tissue, but fat mass is preserved by endogenous synthesis in mice deficient in adipose tissue lipoprotein lipase. Proceedings of the National Academy of Sciences of the United States of America 94: 10261-10266. Link: https://tinyurl.com/yc7plbps
- Lund SA, Giachelli CM, Scatena M (2009) The role of osteopontin in inflammatory processes. Journal of cell communication and signaling 3: 311-22. Link: https://tinyurl.com/ycrj2xt8
- Wei J, Karsenty G (2015) An overview of the metabolic functions of osteocalcin. Reviews in endocrine & metabolic disorders 16: 93-98. . Link https://tinyurl.com/ya8onuoa
- Declercq HA, Verbeeck RM, De Ridder LI, Schacht EH, Cornelissen MJ (2005) Calcification as an indicator of osteoinductive capacity of biomaterials in osteoblastic cell cultures. Biomaterials 26: 4964-4974. Link: https://tinyurl.com/ybkazr47
- Noort WA, Oerlemans MI, Rozemuller H, Feyen D, Jaksani S, et al. (2012) Human versus porcine mesenchymal stromal cells: phenotype, differentiation potential, immunomodulation and cardiac improvement after transplantation. Journal of cellular and molecular medicine 16: 1827-1839. Link: https://tinyurl.com/ya6zluyb
- Brevini TA, Antonini S, Cillo F, Crestan M, Gandolfi F (2007) Porcine embryonic stem cells: Facts, challenges and hopes. Theriogenology 68 Suppl 1: S206-213. Link: https://tinyurl.com/ydbjof78
- Zhang S, Bai C, Zheng D, Gao Y, Fan Y, et al. (2016) Identification and characterization of pig adipose-derived progenitor cells. Canadian journal of veterinary research = Revue canadienne de recherche veterinaire 80: 309-317. Link: https://tinyurl.com/ycfsqnts
- Ock SA, Jeon BG, Rho GJ (2010) Comparative characterization of porcine mesenchymal stem cells derived from bone marrow extract and skin tissues. Tissue engineering. Part C, Methods 16: 1481-1491. Link: https://tinyurl.com/yav458of
- Kang EJ, Lee YH, Kim MJ, Lee YM, Kumar BM, et al. (2013) Transplantation of porcine umbilical cord matrix mesenchymal stem cells in a mouse model of Parkinson's disease. Journal of tissue engineering and regenerative medicine 7: 169-182. Link: https://tinyurl.com/y7qzpkmb
- Kumar BM, Yoo JG, Ock SA, Kim JG, Song HJ, et al. (2007) In vitro differentiation of mesenchymal progenitor cells derived from porcine umbilical cord blood. Molecules and cells 24: 343-350. Link: https://tinyurl.com/y8lu84dd
- Kang EJ, Byun JH, Choi YJ, Maeng GH, Lee SL, et al. (2010) In vitro and in vivo osteogenesis of porcine skin-derived mesenchymal stem cell-like cells with a demineralized bone and fibrin glue scaffold. Tissue engineering. Part A 16: 815-827. Link: https://tinyurl.com/yb859g6a
- Park BW, Kang DH, Kang EJ, Byun JH, Lee JS, et al. (2012) Peripheral nerve regeneration using autologous porcine skin-derived mesenchymal stem cells. Journal of tissue engineering and regenerative medicine 6: 113-124. Link: https://tinyurl.com/ybvj27yq
- Lermen D, Gorjup E, Dyce PW, von Briesen H, Muller P (2010) Neuro-muscular differentiation of adult porcine skin derived stem cell-like cells. PloS one 5: e8968. Link: https://tinyurl.com/yck6jww8
- Rostovskaya M, Anastassiadis K (2012) Differential expression of surface markers in mouse bone marrow mesenchymal stromal cell subpopulations with distinct lineage commitment. PloS one 7: e51221. Link: https://tinyurl.com/yctn8vz8
- Bosnakovski D, Mizuno M, Kim G, Ishiguro T, Okumura M, et al. (2004) Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells in pellet cultural system. Experimental hematology 32: 502-509. Link: https://tinyurl.com/y94edjfn
- Du MH, Y Lu NS, Shu G, Zhu X, Wang L, et al. (2014) Characterization and differentiation into adipocytes and myocytes of porcine bone marrow mesenchymal stem cells. J Integrative Agriculture 13: 837-848. Link: https://tinyurl.com/ydalupty
- Bruckner S, Tautenhahn HM, Winkler S, Stock P, Dollinger M, et al. (2014) A Fat option for the pig: hepatocytic differentiated mesenchymal stem cells for translational research. Experimental cell research 321: 267-275. Link: https://tinyurl.com/y86hgzo8
- Bruckner S, Tautenhahn HM, Winkler S, Stock P, Jonas S, et al. (2013) Isolation and hepatocyte differentiation of mesenchymal stem cells from porcine bone marrow--"surgical waste" as a novel MSC source. Transplantation proceedings 45: 2056-2058. Link: https://tinyurl.com/y99jkcry
- Caminal M, Velez R, Rabanal RM, Vivas D, Batlle-Morera L, et al. (2017) A reproducible method for the isolation and expansion of ovine mesenchymal stromal cells from bone marrow for use in regenerative medicine preclinical studies. Journal of tissue engineering and regenerative medicine 11: 3408-3416. Link: https://tinyurl.com/yat8ygej
- Desantis S, Accogli G, Zizza S, Mastrodonato M, Blasi A, et al. (2015) Ultrastructural study of cultured ovine bone marrow-derived mesenchymal stromal cells. Annals of anatomy = Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft 201: 43-49. Link: https://tinyurl.com/ycr4xwuo
- Rentsch C, Hess R, Rentsch B, Hofmann A, Manthey S, et al. (2010) Ovine bone marrow mesenchymal stem cells: isolation and characterization of the cells and their osteogenic differentiation potential on embroidered and surface-modified polycaprolactone-co-lactide scaffolds. In vitro cellular & developmental biology. Animal 46: 624-634. Link: https://tinyurl.com/ycfh5sxk
- Adamzyk C, Emonds T, Falkenstein J, Tolba R, Jahnen-Dechent W, et al. (2013) Different Culture Media Affect Proliferation, Surface Epitope Expression, and Differentiation of Ovine MSC. Stem cells international 2013, 387324. Link: https://tinyurl.com/yc7yavjh
- McCarty RC, Gronthos S, Zannettino AC, Foster BK, Xian CJ (2009) Characterisation and developmental potential of ovine bone marrow derived mesenchymal stem cells. J cellular physiol 219: 324-333. Link: https://tinyurl.com/ycdz68bt
- Boos AM, Loew JS, Deschler G, Arkudas A, Bleiziffer O, et al. (2011) Directly auto-transplanted mesenchymal stem cells induce bone formation in a ceramic bone substitute in an ectopic sheep model. J cellular molecular med15: 1364-1378. Link: https://tinyurl.com/yaoo4nft
- Weber B, Kehl D, Bleul U, Behr L, Sammut S, et al. (2016) In vitro fabrication of autologous living tissue-engineered vascular grafts based on prenatally harvested ovine amniotic fluid-derived stem cells. Journal of tissue engineering and regenerative medicine 10: 52-70. Link: https://tinyurl.com/ybg662uq
- Martinez-Lorenzo MJ, Royo-Canas M, Alegre-Aguaron E, Desportes P, Castiella T, et al. (2009) Phenotype and chondrogenic differentiation of mesenchymal cells from adipose tissue of different species. Journal of orthopaedic research: official publication of the Orthopaedic Research Society 27: 1499-1507. Link: https://tinyurl.com/yao9pzml
- Sanjurjo-Rodriguez C, Castro-Vinuelas R, Hermida-Gomez T, Fernandez-Vazquez T, Fuentes-Boquete IM, et al. (2017) Ovine Mesenchymal Stromal Cells: Morphologic, Phenotypic and Functional Characterization for Osteochondral Tissue Engineering. PloS one 12: e0171231. Link: https://tinyurl.com/y8rhzmw5
- De Schauwer C, Meyer E, Van de Walle GR, Van Soom A (2011) Markers of stemness in equine mesenchymal stem cells: a plea for uniformity. Theriogenology 75: 1431-1443. Link: https://tinyurl.com/ycaxjcjp
- Tian Y, Tao L, Zhao S, Tai D, Liu D, et al. (2016) Isolation and morphological characterization of ovine amniotic fluid mesenchymal stem cells. Experimental animals 65: 125-134. Link: https://tinyurl.com/y7st7kmg
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
