International Journal of Veterinary Science and Research
Factors affecting Somatic Cell Count in milk of dairy cows in Costa Rica
1Department of Molecular Environmental Biology, University of California, Berkeley, USA
2Education Abroad Program, University of California, Monteverde, Costa Rica, USA
3University of California Education Abroad Program, Tropical Biology and Conservation Program, Costa Rica, USA
Author and article information
Cite this as
Kline KE, Flores S, Joyce F (2018) Factors affecting Somatic Cell Count in milk of dairy cows in Costa Rica. Int J Vet Sci Res. 2018; 4(1): 001-008. Available from: 10.17352/ijvsr.000027
Copyright License
© 2018 Kline KE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Somatic cells, such as bacteria fi ghting leukocytes, are present in cow’s milk as an immune response to combat intramammary infection. The primary objective of this study was to determine the effect of various factors on the somatic cell count, or SCC, within the milk of dairy cows. Factors studied in comparison to the SCC include the number of bacteria within the cows’ milk, the age of the cows, the number of Hypoderma lineatum lesions on the cows, and the cows’ diet and the sanitation practice at separate farms. Milk samples were collected from 13 cows from Farm 1, located in La Cruz, Costa Rica, 20 cows from Farm 2, located in La Cruz, Costa Rica, and 30 cows from Farm 3, located in Monteverde, Costa Rica. Farmers at each of these three farms fed their cows a slightly different diet, and carried out different procedures in relation to cleanliness during their milking process. The stage of lactation was kept constant to eliminate this confounding variable. Milk samples were observed for somatic cell and bacteria counts using a compound light microscope under 1000X magnifi cation using gel immersion. The fi ndings from this study indicate that somatic cells are signifi cantly correlated to bacteria in cow’s milk, while somatic cells do not signifi cantly correlate with the cow’s age or the number of H. lineatum lesions a cow has. This study also indicates that the sanitation practices and milking procedure that farmers use can affect their somatic cell counts, and that using a new and disposable cotton cloth or paper to wipe down the udders before milking may lead to lower somatic cell and bacteria counts than using the same wet rag to wipe down every cow. Also, treating the udders with a disinfectant before milking, instead of after, led to the lowest somatic cell and bacteria count.
In the last three decades, world milk production has increased by more than 50 percent, with developing countries broadening their share in global dairy production in more recent years [1]. Both the income of dairy operations and the health of the consumers depend on a healthy composition of the milk that is processed and sold. It is propitious that milk has low levels of deleterious compounds such as somatic cells or bacteria: high levels of somatic cells in milk can hinder the production of other dairy products, and high levels of bacteria in milk can pose health risks for consumers [2]. Regulatory standards are set forth worldwide to determine the healthy limits of somatic cell and bacteria that can be present in milk before it can be accepted from farms and processed. These regulatory standards vary between countries, but in all locations, milk is tested in a laboratory after being collected from farms for a bacteria count and somatic cell count [3]. The bacteria count is a detection of microbial contamination, and is generally used to evaluate the sanitation of the dairymen’s equipment and the overall health of the herd [4]. In the United States, the Grade A Pasteurized Milk Ordinance (PMO) requires bacteria to be less than 100,000 bacteria per mL for Grade A milk shipments. This would be indicated by finding a count of five bacteria in 20 total fields of view from a milk sample on a microscope slide under 1000X magnification. In Costa Rica, it must meet the standardized guidelines outlined by the Servicio Nacional de Sanidad y Calidad Agroalimentaria (SENASA) through the Ministry of Agriculture, which also requires the bacteria to be less than 100,000 bacteria per mL (indicated by a count of five bacteria on a microscope slide). Somatic cell count, or SCC, is also an indicator of the quality of milk, and generally a lower SCC indicates better animal health [5]. In the United States, the current legal limit put forth by the PMO is 750,000 somatic cells per mL for Grade A milk, which would be indicated by finding a count of 37 somatic cells from 20 total fields of view on a microscope slide under 1000X magnification [6]. In Costa Rica, there must be less than or equal to 400,000 somatic cells per mL according to SENASA, which would be indicated by finding a count of 20 somatic cells from a milk sample on a microscope slide under 1000X magnification. Milk from an uninfected mammary gland usually contains less than 100,000 cells per mL, and a milk SCC of more than 200,000 cells per mL may suggest that an inflammatory response has been elicited or that the cow is recovering from infection [7].
The somatic cells present in the milk of lactating cows consist of leukocytes, or white blood cells, from the cows immune system and secretory cells (shed from the udder tissue). Leukocytes play an important role in the body for mediating immune responses against infectious microorganisms [2]. Just as nutrients are transported from the bloodstream into the udder of lactating cows to be converted into milk, leukocytes will migrate from blood into the mammary gland through the teat canal in response to bacteria within the udder. Such infecting bacteria in the udder multiply and can cause intramammary infection, leading to tissue inflammation and damage. Neutrophils are involved in repairing this damage and destroying bacteria in the udder tissue [7].
The difference in somatic cell counts between cows can result from differences in physiological factors and the functioning of each cow’s immune system [8]. A compromised immune system can make an animal more susceptible to infection, and the efficacy of an immune response is best measured by how quickly the immune system reacts to an infectious challenge.
One factor that can affect the health and immune system of dairy cows is parasitic flies such as H. lineatum. These organisms cause furuncular myiasis in cows, which is an infestation of a vertebrate host by fly larvae that feed on living tissue, body fluids, or ingested foods. Following copulation, an adult H. lineatum will capture a mosquito or a small blood-feeding fly and transfer its larva. When this mosquito or small fly feeds on the body of a cow, the larvae are transferred to the subcutaneous layer of the cow. The larvae then act as parasites by existing under the subcutaneous layer of the cow’s skin. This produces myiasis, which appears as boil-like lesions or open wounds on the outer surface of the cow’s body. These lesions can get scratched and inflamed, leading to a secondary infection by the infestation of bacteria. When the larvae develop, they fall to the ground, burrow, pupate, and then molt into an adult fly. These flies can transfer infections to animals and can cause cows to be more susceptible to diseases. The objective of this study was to investigate factors affecting SCC in milk of cows in Costa Rica. Factors evaluated included the number of bacteria, age, the sanitation and milking practices of the farm, the diet of the cow, and the number of H. lineatum lesions on the cow. It was predicted that an increase in the number of bacteria within the milk, an increase in H. lineatum lesions on the cow, and less meticulous sanitation practices on the farm would correlate with an increase in SCC in the milk.
Materials and Methods
The first step in the experiment was to obtain milk samples from cows with a range of ages, a range in the number of H. lineatum lesions on their body, and from farms that practice different feeding and milking practices. Milk was collected from 13 cows at one farm, 20 cows from a second farm, and 30 cows from a third farm, all of which were during the second (afternoon) milking session of the day. The stage of lactation was kept constant to eliminate this confounding variable.
Farm 1
The first farm was in La Cruz, Costa Rica. The cows at this farm switched off feeding on three pastures throughout the day, each one having a different type of grass: one with Cynodon nlemfuensis, one with Brachiaria brizantha, and one with Pennisetum purpureum. The cows were also supplemented with a food concentrate and a mineral supplement.
For the milking procedure at this farm, the farmer brought each cow into a stable, rinsed the udders with water, and wiped the udders off with a wet, cotton cloth. He used this same wet cloth to wipe the udders of each cow before milking them. The farmer released several squirts of milk from the udders with his hand to eliminate the foremilk and check for any clotting before milking. He then disinfected the udders with a solution that is 1% iodine after the milking is completed. The farmer used this order of procedures to help collect the milk samples from each cow.
After each milk sample was obtained, a permanent marker was used to label each plastic bag with the number on the cow’s ear tag. The labeled sample was secured and placed it in ice to keep the milk under 40 degrees Fahrenheit in order to eliminate bacterial growth [9]. During this time, the cow’s entire external body was examined for H. lineatum lesions and this number, as well as the cow’s age, was counted and recorded. This process was repeated for all 13 cows at this farm.
Farm 2
The second farm was in La Cruz, Costa Rica. The cows at this farm fed on Cynodon nlemfuensis and Saccharum officinarum, and were also supplemented with a food concentrate and a mineral supplement. For the milking procedure at this farm, the farmer first cleaned the udders by dipping each one in a solution containing 1% iodine in order to disinfect them. He then wiped and dried the udders with a piece of newspaper. He uses a new piece of dry newspaper for each cow. The farmer then released several squirts of milk into a strip cup to release the foremilk and check for any clotting in the milk before beginning the milking process. The farmer used this order of procedures to help collect the milk samples from each cow. Each bag was labeled with the cow’s ear tag number before placing it in ice to keep the samples under 40 degrees Fahrenheit. Each cow’s entire body was then observed for H. lineatum lesions and this number and the cow’s age was recorded next to the cow’s ear tag number in a notebook. This process was repeated for all 20 cows at this farm.
Farm 3
The third farm was in Monteverde, Costa Rica. The cows at this farm were fed only Cynodon nlemfuensis grass, and were supplemented with a food concentrate and a mineral supplement. For the milking procedure at this farm, the farmer rinsed the udders with water from a hose, while wiping them off with his hand. He then wiped the udders off with a wet, cotton cloth, while also releasing a few initial squirts of the foremilk with this cloth. He used a new, wet cloth from a bucket of water for each cow. After the milking is complete, he disinfected the udders with a 1% iodine solution. The farmer used this order of procedures to help collect the milk samples from each cow.
Each plastic bag was labeled with the cow’s ear tag number and placed it in ice to keep the sample under 40 degrees Fahrenheit. The cow’s entire body was observed for H. lineatum lesions, and this number was recorded as well as the cow’s age next to the cow’s ear tag number in a notebook. This process was repeated for all 30 cows on this farm.
Counting somatic cells and bacteria
In order to decipher the somatic cell count and the bacteria cell count of the milk samples, the standard methodology practiced within the laboratory at the Monteverde Cheese Factory, located in Monteverde, Costa Rica, to evaluate the milk they receive from farms before the milk is processed was used. Sterile Pasteur pipette was used to transfer ten microliters of milk from each plastic bag onto a glass microscope slide. Before transferring the milk with the Pasteur pipette, each plastic milk sample bag was shook to mix the milk thoroughly before testing it. Each slide contained four ten microliter drops, each coming from a different milk sample. The drops were labeled with the corresponding ear-tag number from the cow that the sample was taken from. Using the tip of the pipette, ten microliters of milk was spread on the slide into a thin-layered circle. The slides, which had four drops each, were placed under a light bulb for ten minutes to dry. Once the milk was dry, the slides were placed in a methylene blue dye for five minutes in order to stain the somatic cells and bacteria. After five minutes, the excess dye was drained by placing the slides on their sides on absorbent paper. Once the slides were free of excess dye, they were dipped in distilled water and placed them under a light bulb for five minutes until they were completely dry.
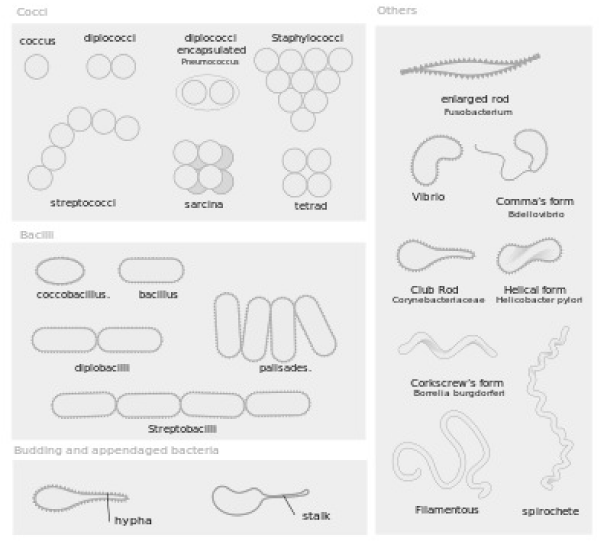
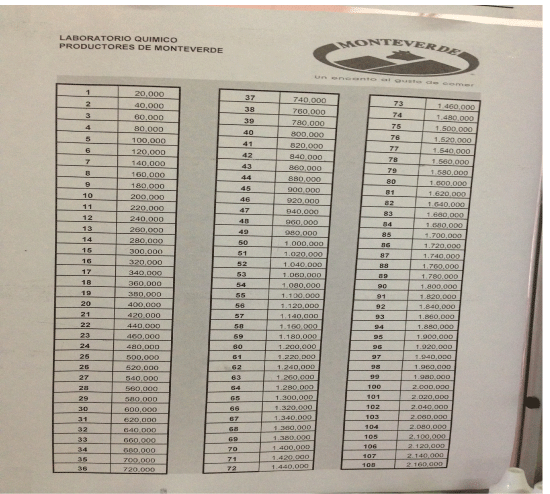
Using a compound light microscope, the number of somatic cells and bacteria were counted in 20 different fields of view for each cow’s sample (Figure 1). The slides were observed under 1000X magnification using oil immersion. The somatic cells were detected by observing dark blue spots that were greater than eight microns in size and possess a stained nucleus. Cellular fragments less than four microns in size were not counted as a somatic cell, and cells that were clumped together were counted as one cell unless two distinct nuclei were able to be detected. Bacteria were much smaller than the somatic cells, and were in one of the following morphological forms depicted in figure 2: coccus, dicoccus, streptococci, coccobacillus, bacillus, or streptobacilli. An average coccus bacteria is 0.5-1.0 micrometers in diameter. In some cases, coccus or coccobacillus would be in colonies, the colony was counted as one bacteria (as practiced within the laboratory at the Monteverde Cheese Factory). After counting the number of somatic cells and bacteria in all 20 fields of view, the chart shown in figure 3 was used, which indicates how these values correspond to the total number of somatic cells and bacteria in the entire cow. For example, one somatic cell count or one bacteria count found in the twenty fields of view within the drop of milk on the slide corresponds to 20,000 somatic cells or bacteria in the entire cow, while a count of four somatic cells or four bacteria found on the slide corresponds to 80,000 somatic cells or bacteria in the entire cow.
With JMP Statistical Package, Anova was used to compare the average number of bacteria, somatic cells, and H. lineatum lesions from all three farms.
Results
For the 13 cows at Farm 1, there was an average of 11.07 H. lineatum lesions on each cow (SD= 14.79). The highest was a cow having 50 H. lineatum lesions, and the lowest was a cow with none. Farm 1 had an average somatic cell count of 40.31 cells/ml (SD= 57.31 cells/ml). Each one count corresponds to 20,000 somatic cells in the entire cow, according to figure 3, so this average corresponds to 806,000 total somatic cells. The highest was a cow having a somatic cell count of 141 cells/ml, and the lowest was a cow having a somatic cell count of one cell/ml. Farm 1 had an average bacteria count of 11.54 bacteria/ml (SD= 10.54 bacteria/ml). Each one count corresponds to 20,000 total bacteria in the cow, according to figure 3, so this average corresponds to 230,800 total bacteria. The highest was a cow having a bacteria count of 32 bacteria/ml, and the lowest was a cow having a bacteria count of zero bacteria/ml.
For the twenty cows at Farm 2, there was an average of 2.4 H. lineatum lesions on each cow (SD= 2.68). The highest was a cow having ten H. lineatum lesions, and the lowest was a cow with none. Farm 2 had an average somatic cell count of 9.6 cells/ml (SD= 8.04 cells/ml). According to figure 3, this average corresponds to 192,000 total somatic cells. The highest was a cow having a somatic cell count of 35, and the lowest was a cow having a somatic cell count of zero. Farm 2 had an average bacteria count of 5.4 bacteria/ml (SD= 4.5 bacteria/ml). According to figure 3, this average corresponds to 108,000 total bacteria. The highest was a cow having a bacteria count of 14 bacteria/ml, and the lowest was a cow having a bacteria count of zero bacteria/ml.
For the 30 cows at Farm 3, there was an average of 0.23 H. lineatum lesions on each cow (SD= 0.50). The highest was a cow having one H. lineatum lesion, and the lowest was a cow with none. Farm 3 had an average somatic cell count of 15.17 cells/ml (SD= 18.25 cells/ml). This average corresponds to 303,400 total somatic cells in the entire cow. The highest was a cow having a somatic cell count of 90 cells/ml, and the lowest was a cow having a somatic cell count of zero cells/ml. Farm 3 had an average bacteria count of 3.8 bacteria/ml (SD= 4.5 bacteria/ml). This average corresponds to 76,000 total bacteria. The highest was a cow having a bacteria count of ten bacteria/ml, and the lowest was a cow having a bacteria count of zero bacteria/ml.
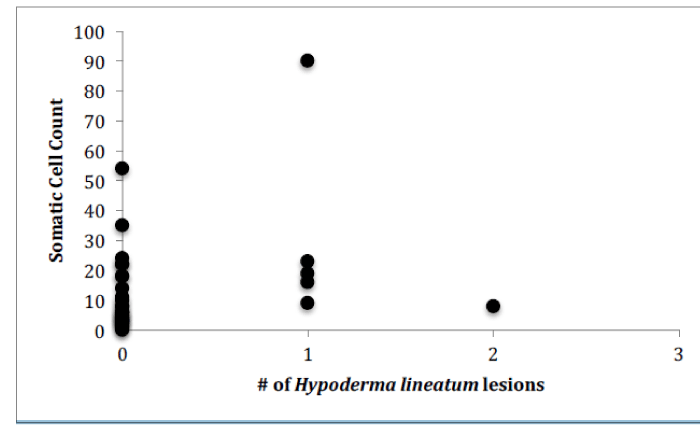
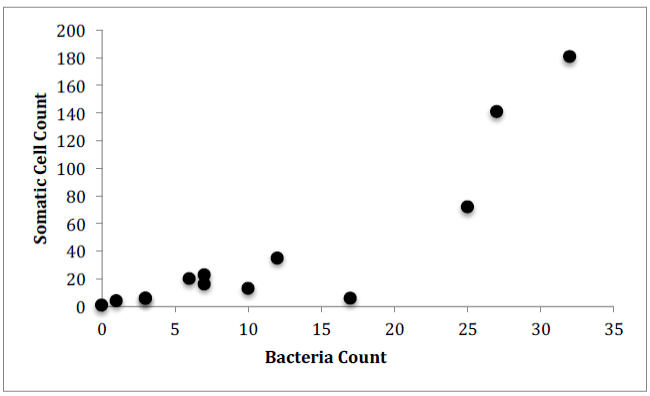
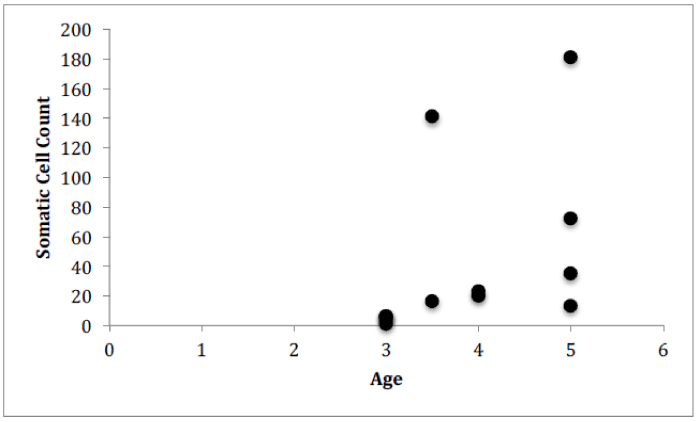
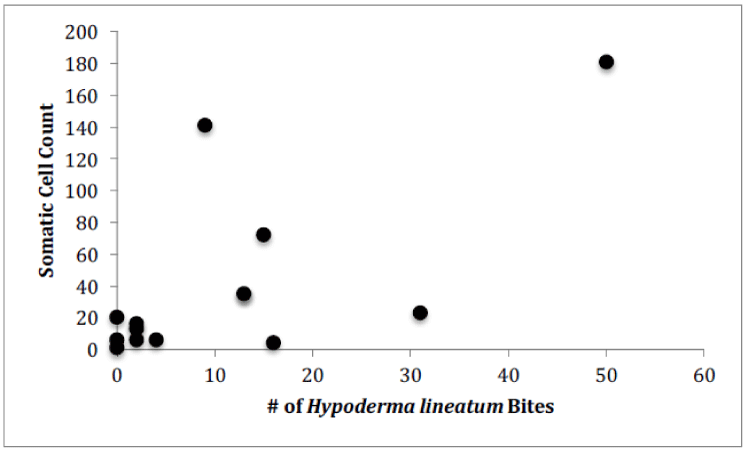
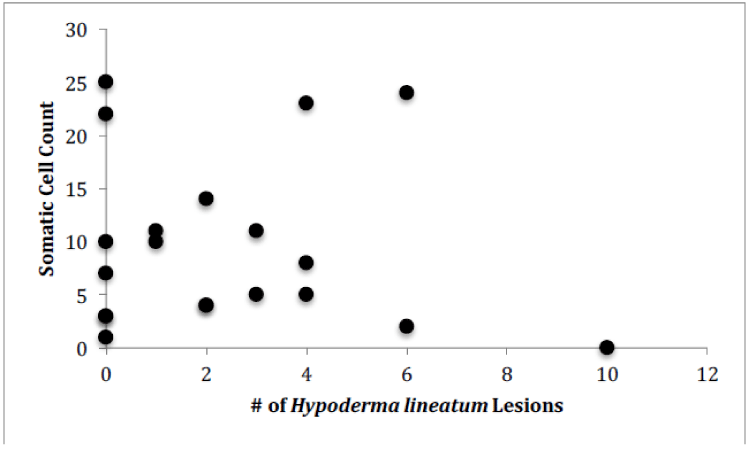
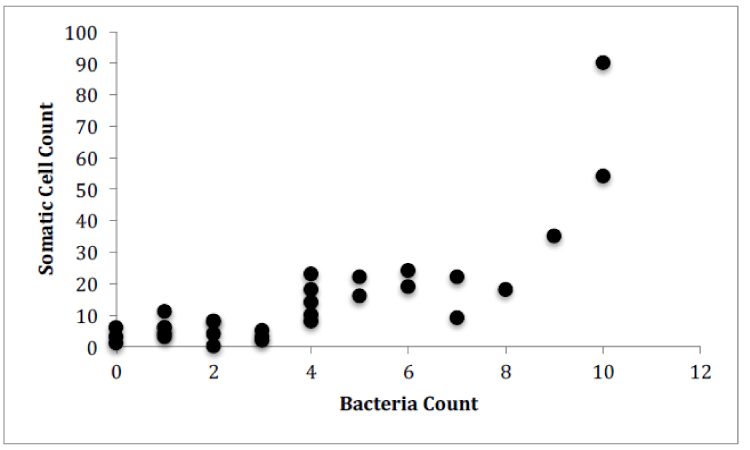
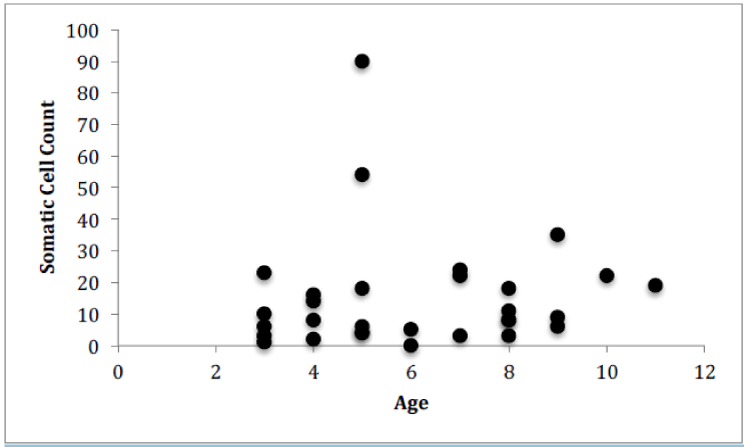
For Farm 1, there was a significant correlation between the number of somatic cells and the number of bacteria for each of the cows (Figure 4, Pearson correlation, r(12)=0.7888, p<0.0001). There was no significant correlation between the number of somatic cell and the age of each cow (Figure 5) or the number of somatic cells and the number of H. lineatum lesions (Figure 6).
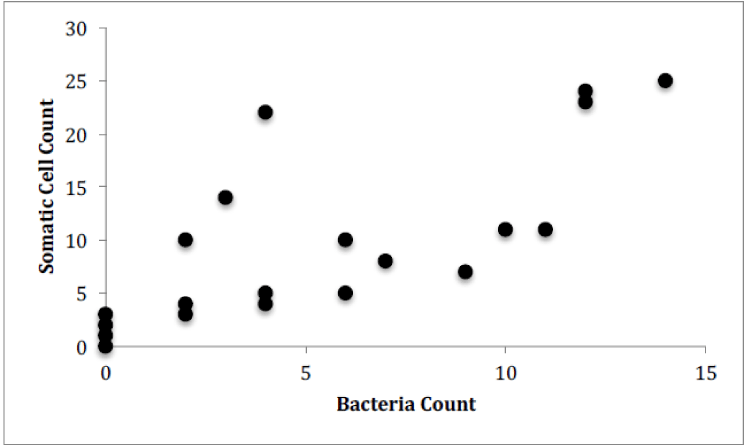
For Farm 2, there was a significant correlation between the number of somatic cells and the number of bacteria for each of the cows (Figure 6, Pearson correlation, r(19)=0.555, p=0.0002). There was no significant correlation between the number of somatic cell and the age of each cow (Figure 7) or the number of somatic cells and the number of H. lineatum lesions (Figure 8).
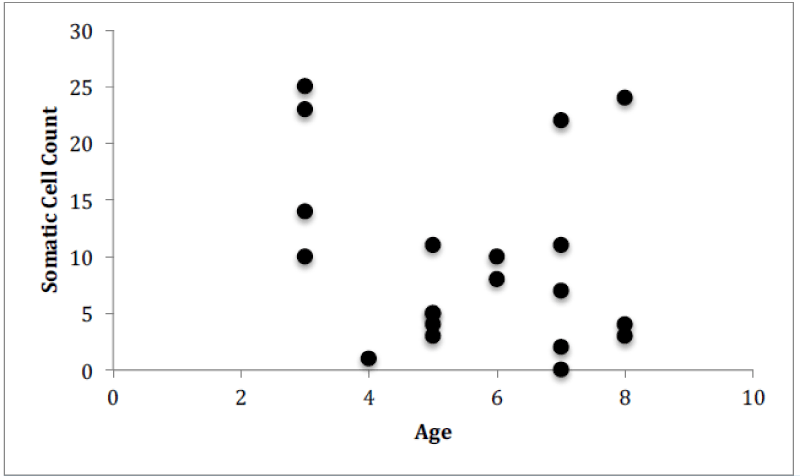
For Farm 3, there was a significant correlation between the number of somatic cells and the number of bacteria for each of the cows (Figure 9, Pearson correlation, r(29)=0.615, p<0.0001). There was no significant correlation between the number of somatic cell and the age of each cow (Figure 10) or the number of somatic cells and the number of H. lineatum lesions (Figure 11).
The cows from Farm 1 had a significantly higher number of somatic cells than the cows from both Farm 2 and Farm 3 (Table 1, F2,62= 4.844, p= 0.0112). The cows from Farms 2 and 3 did not have a significantly different number of somatic cells. The cows from Farm 1 had a significantly higher number of bacteria than both Farm 2 and Farm 3 (Table 1, F2,62= 8.28, p= 0.0007). The cows from Farms 2 and 3 did not have a significantly different number of bacteria. The cows from Farm 1 also had a significantly higher number of H. lineatum lesions than both Farm 2 and Farm 3 (Table 1, F2,62= 11.74, p< 0.0001). The cows from Farms 2 and 3 did not have a significantly different number of H. lineatum lesions.
Discussion
Somatic cells vs. Bacteria
These results show a significant correlation between the number of somatic cells and the number of bacteria in cows’ milk. This might indicate that when infection is present in the udders, and a higher amount of bacteria penetrate the physical barrier of the udder canal, an influx of leukocytes, in the form of somatic cells, enter the udders as an immune response to fight these infectious microorganisms. Even though somatic cells are constantly circulating in the blood, the body sends a high number of them from the blood stream into the udders when infection is present [10]. The Somatic cell count can therefore be an indicator of udder health, because it can indirectly detect intramammary infection in dairy cows; as bacteria numbers in the udders increase, somatic cell numbers also increase [11].
This is crucial for people in the dairy industry, because the production of maximum quantities of high quality milk is the most important goal of every dairy operation. An increase in somatic cells in cows’ udders can lower a farmer’s milk yield, and therefore income. Inflammation and infection of the udders is considered to be the disease that has the greatest financial impact on the dairy industry [11]. Economic losses of mastitis in dairy cows include the cost to treat the infection, reduced milk yield, changes in milk composition, or premature culling, which is the separation of particular animals from the herd based on specific criteria [11]. Milk cannot be processed or sold when somatic cell and bacteria counts are higher than the legal limits, such as those previously stated that are put forth by the PMO in the United States and SENASA in Costa Rica. This means that if somatic cell and bacteria counts in the milk coming from a farm are higher than these regulated limits, the farmers will experience economic losses.
Milk is produced in specialized cells in the alveoli of the cow’s udders. Increased somatic cells lead to decreased milk yield because when many leukocytes move into the udders, they push out milk secreting cells, making the alveoli less productive in producing milk [5]. Inflammation resulting from higher bacteria in the udders also leads to compromised secretion function and altered volume and composition of milk secreted. Accordingly, it is imperative for farmers to use practices that work to thwart intramammary infections in their cows, because such infections increase somatic cell counts in the milk, lower the total milk yield, and therefore decrease the farmer’s income.
Somatic cells vs. Age
Although previous research has shown that when age increases, the SCC increases [12], data from this study indicated that an increase in a cow’s age does not correlate to a higher number of somatic cells in the milk. The older cows did not display more bacteria in their milk, or more somatic cells fighting off those bacteria. These findings show that the age of a cow does not necessarily affect the functioning of the cow’s immune system, and older cows do not display a greater chance of contracting an intramammary infection.
Somatic cells vs. H. lineatum lesions
Although previous studies [13], have shown that cows with higher numbers of parasites, particularly ticks, have shown higher numbers of somatic cells in their milk, results from this study indicate that cows with higher numbers of H. lineatum lesions on their body did not correlate with higher a somatic cell count in their milk. Affected regions where the fly larva exist in the subcutaneous layer of the cow’s skin cause a lesion that can get scratched, irritated, and inflamed, making it more prone to secondary infections by bacteria. These flies can also transmit diseases to cows, and can secrete enzymes that have been suggested to negatively affect the cows’ immune system. Although such factors can have a potentially overwhelming effect on the cow’s immune system, data from this study indicated that this effect is not large enough to increase the cow’s chance of developing an intramammary infection. Therefore, according to these results, cows with more H. lineatum lesions do not necessarily have more bacteria in their milk, or more somatic cells fighting off those bacteria.
Notwithstanding of this finding, farmers should still treat H. lineatum wounds, and measures should be taken to prevent further lesions. This is because of the economic losses that the flies still render, and the health effects that they still have on cows. Oviposition by the adult H. lineatum can cause cattle to gad (a wild running behavior to seek shelter), which can lead to weight loss and a decrease in milk production by up to ten percent due to interrupted grazing patterns. The host response to the presence of H. lineatum larvae in their tissue is decreased tendency to gain weight, and a rash or irritated area of the skin where the lesion is: leading to further damage and possible infection. Insecticides or broad-spectrum parasiticides are available to treat and prevent these parasitic flies in cattle. Insecticide-impregnated ear tags slowly release low levels of chemicals that offer protection from the flies for extended periods. Another device that offers slow release of insecticides are insecticide-impregnated ankle bands that can be attached around the back legs of cows. Farmers can also inject insecticides or parasiticides into the back of cows, or use low-volume sprays applied to the entire surface of the cow’s body [14].
Differences between the Farms
These results indicate differences in the numbers of H. lineatum lesions, somatic cell counts, and bacteria counts between the three farms that were analyzed. These three farms had different feeding and milking practices. The first farm showed a significantly higher average number of somatic cells, bacteria, and H. lineatum lesions than the other two farms, but Farms 2 and 3 did not show a significant difference in these factors between each other. The somatic cell count and bacteria count for Farm 1 was above the regulatory standards for healthy legal limits in milk put forth by both the PMO for the United States and SENASA for Costa Rica, while the somatic cell and bacteria count for Farms 2 and 3 were under healthy limits.
The main difference in the milking procedure was that at Farm 1, the farmer did not use a disposable cotton cloth or paper to wipe down each cow’s udders before milking, but used the same wet rag for every cow. The farmer at Farm 3 used a different wet rag for each cow, and the farmer at Farm 2 used a new piece of dry newspaper for each cow. This information displays the importance in using a new or disposable cotton cloth or piece of newspaper for each cow to wipe the udders before milking, as practiced at Farms 2 and 3, instead of the same cotton cloth for each cow, as practiced at Farm 1, in order to keep the somatic cell and bacteria counts low and under healthy, legal limits. An article in Medvet, which focuses on industry health and safety, testing, training, and prevention, gives the proper steps that should be taken in the milking process in order to reduce and maintain a low somatic cell count [15]. Prior to milking, this article emphasizes the need to dry the teats completely using individual clean cloth or paper towel, and to avoid getting the rest of the udder wet [15]. The farmers at both Farm 1 and Farm 3 contradicted these recommendations by rinsing the udders completely with water before milking, and by using wet cloths to wipe the udders off instead of drying them before milking. The farmer at Farm 1, with the highest average somatic cell and bacteria counts, also went against these recommendations by not using an individual clean cloth for each cow. The farmer at Farm 2 on the other hand, did not rinse the udders with water, and used a new piece of newspaper to completely dry the udders after disinfecting them before milking. This was the farm with the lowest somatic cell count on average. Farm 2 was also the only farm where the farmer used the iodine solution as a disinfectant before milking, as opposed to after milking such as at Farms 1 and 3. A study done on the effect of pre-milking teat preparation found similar results on the benefits of using a disinfectant before milking in reducing bacteria; this study demonstrated that the use of some disinfectant products for pre-milking teat preparation can have reduce the levels of staphylococcal and streptococcal pathogens on cow’s udders [16].
Results from this study display how the milking procedure and sanitation practice that a farmer uses greatly determines the health of the cows, and the cows’ susceptibility to infection. These precautious cleanliness procedures may decrease the risk for an intramammary infection in the cows by decreasing harmful microorganisms, leading to lower bacteria in the udders, and therefore less somatic cells in the milk fighting off this bacteria in the udder tissue.
The greatest diet difference between the cows at the different farms was that Farm 2 was the only farm where the farmers fed their cows Saccharum officinarum. Because Farm 2 had the lowest average numbers of H. lineatum lesions, somatic cells, and bacteria, this may indicate that Saccharum officinarum is a sufficient food source for cows that provides the right amount of nutrients to aid the immune system in fighting off destructive microorganisms and defend against infection. Further research can be performed on this specific point by repeating this experiment and comparing cows that eat solely Saccharum officinarum compared to cows that eat other food sources such as Cynodon nlemfuensis, Brachiaria brizantha, and Pennisetum purpureum grasses [17-25].
Gratitude goes out to the farmers, Evelio Vargas, Alfredo and Diego Vargas, and Joe Stuckey for allowing milk samples to be obtained from their farms for this study.
Also, gratitude goes out to the workers at the Monteverde Cheese Factory located in Monteverde, Costa Rica for lending their support in learning how to count somatic cells and bacteria under a microscope, and for lending some necessary equipment. Finally, gratitude goes out to Sophia Flores for assisting in arranging the trips to these farms and to the cheese factory laboratory.
- (2015) 'Dairy Production and Products.' Dairy Production and Products. Food and Agriculture Organization of the United Nations, 2015. Web. 4 June 2015.
- (2005) Human Health Risks Associated With High Somatic Cell Count Milk. National Mastitis Council Annual Meeting Proceedings, Jan. 2005. Web. 26 Apr. 2015. Link: https://goo.gl/tbBMSB
- Oliver SP (2010) How Milk Quality Is Assessed. Extension 21197. Link: https://goo.gl/Py5kwd
- (2015) Laboratory Test Procedures for Raw and Retail Milk. Net Health. Northeast Texas Public Health District, 2015.
- 'The Basics of Somatic Cell Counts and What They Mean.' All about Somatic Cell Counts. Irish Cattle Breeding Federation, n.d. Web. 26 Apr. 2015.
- (2012) Determining U.S. Milk Quality Using Bulk-tank Somatic Cell Counts, 2011. Veterinary Services. Animal and Plant Health Inspection Services. Link: https://goo.gl/ec6p9a
- Harmon, Robert J (2001) Somatic Cell Counts: A Primer. AGWEB. National Mastitis Council Annual Meeting Proceedings, 2001. Web. 27 Apr. 2015.
- Lee, David L (2015) Factors Influencing Somatic Cell Count Variation in Cow Milk Samples. Cooperative Extension, n.d. Web. 27 Apr. 2015.
- (2006) Sampling Producer Milk Circular 278. Publication. Albany: New York State Department of Agriculture and Markets Division of Milk Control and Dairy Services. Link: https://goo.gl/kixaBn
- Escobar EN (1994) Somatic Cells in Goat Milk. Link: https://goo.gl/usoZMm
- Spanu C, Ym Berger, Dl Thomas and Pl Ruegg (2011) Impact of Intramammary Antimicrobial Dry Treatment and Teat Sanitation on Somatic Cell Count and Intramammary Infection in Dairy Ewes. Small Ruminant Research 97: 139-145. Link: https://goo.gl/MJWsN8
- Sabuncu AH (2013) The Effect of Parity, Age, and Season on Somatic Cell Count of Dairy Cows with Subclinical Mastitis. Journal of Animal and Veterinary Advances 12.4: 472-77. Link: https://goo.gl/STxNxT
- Rojas Martinez, Jose Luis, and Anabelle Castro (2004) Algunas Consideraciones Generales Sobre Las Ivermectinas. Buletin De Parasitologia 5: 1.
- Mullen Gary R, Lance A (2002) Durden. Medical and Veterinary Entomology. Link: https://goo.gl/AGsg1o
- (2012) How to Reduce and Maintain Low Somatic Cell Count: Keep an Eye on the Dial. Medvet. UMontreal, June 2012. Web. 26 May 2015.
- Gleeson D, B O'Brien, J Flynn, E O'Callaghan, F Galli (2009) Effect of Pre-milking Teat Preparation Procedures on the Microbial Count on Teats Prior to Cluster Application. Ir Vet J 62: 461-467. Link: https://goo.gl/ujYyhW
- (2015) Bacteria Cellular Morphologies. Digital image. Bacteria Cellular Morphologies. Wikipedia. Link: https://goo.gl/R6Sozm
- Dohoo IR, AH Meek (1982) Somatic Cell Counts in Bovine Milk. Can Vet 23: 119-125. Link: https://goo.gl/C2KHz2
- Gurmessa, Jamila, Achenef Melaku (2012) Effect of Lactation Stage, Pregnancy, Parity and Age on Yield and Major Components of Raw Milk in Bred Cross Holstein Friesian Cows. World Journal of Dairy & Food Sciences 7: 146-149. Link: https://goo.gl/LddbVH
- (2000) Health Management: Cattle Grub (Warble) Control for Feedlot Cattle. Rep. Alberta Feedlot Management Guide, Sept. 2000.
- (2011) Milk for Manufacturing Purposes and Its Production and Processing. Agricultural Marketing Service. United States Department of Agriculture. Link: https://goo.gl/6V3dtY
- Reneau, Jeffrey K (2001) Somatic Cell Counts: Measures of Farm Management and Milk Quality. National Mastitis Council Annual Meeting Proceedings, 2001.
- Ruegg Pamela L (2002) Milk Quality and Mastitis Tests. 1-33. Link: https://goo.gl/5naZae
- (2006) Secretary of Agriculture, Livestock, Fishing and Food. Quality Protocol For Dulce De Leche (Milk Caramel Argentinian Type). N.p.: Secretary of Agriculture, Livestock, Fishing and Food. Link: https://goo.gl/SoHTtj
- Thompson, Christian F (2008) Dermatobia Hominis (Linnaeus Jr., 1781) (Diptera: Oestridae). United States Department of Agriculture, 21 Aug. 2008. Link: https://goo.gl/gjctRp
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
