International Journal of Veterinary Science and Research
Is Atypical Human Trypanosomosis an Emering Threat to Human Society? : A Debatable one Health Issue to Public Health Experts and Parasitologists
Department of Veterinary Parasitology, College of Veterinary Science, Guru Angad Dev Veterinary and Animal Science University, Ludhiana-141004, Punjab, India
Author and article information
Cite this as
Parashar R, Singla LD, Kaur P (2016) Is Atypical Human Trypanosomosis an Emering Threat to Human Society? : A Debatable one Health Issue to Public Health Experts and Parasitologists. Int J Vet Sci Res. 2016; 2(1): 036-041. Available from: 10.17352/ijvsr.000013
Copyright License
© 2016 Parashar R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Trypanosomosis is caused by different species of unicellular eukaryotic haemofl agellate Trypanosoma. Though human infection by animal species of trypanosomes is “not possible” as these species fails to infect humans due to innate immunity of the host due to presence of trypanolytic factor in human serum, however, across the world 20 patients with atypical human trypanosomosis are documented, eight of which are confi rmed between 1974 and 2014 due to improved molecular diagnostic assays. However, the numbers of cases are atypical human trypanosomosis caused by animal trypanosomes might be underestimated. Ten atypical cases of human trypanosomosis have so far been reported form Indian subcontinent. Out of these, nine cases were from India and one from Sri Lanka.Three cases of human T. evansi have been reported from the Indian subcontinent (one from Sri Lanka and two from India) during the last decade. Apart from these three cases, at least six more atypical human cases of trypanosomosis caused by rat trypanosome, T. lewisi have been reported. Two casualties due to non-tsetse transmitted trypanosomosis (NTTT) were also reported from India. High prevalence of these two animal trypanosomes in India is now a matter of concern for public health specialists. These raise it as an alarming situation of emerging new zoonotic disease and debatable one health issue to public health experts and parasitologist. There is a need to investigate disease with modern diagnostics by epidemiological based surveys in the fi eld to know the exact situation of the disease. Veterinarian can play very crucial role in diagnosis and control of disease, also by educate and re-educate people about the transmission, prevention and control of disease.
The present scenario indicates that by 2050, the human population will reach at such an alarming high level that there will be need of approximately 50% increase in the production of food for the consumption of human beings [1]. This will lead to a situation where survival will be impossible without the animal food for the purpose of balanced ration. At present, animals are at constant risk of infections by bacteria, viruses, fungi and parasites. In addition to this, emerging infectious diseases are day by day becoming curse to animal and human society. These new emerging infectious diseases are causing serious imbalances in stability of the ecosystem, resulting in disturbances of ecological cycles [2-4]. The environmental conditions in India are conducive to the spread of the parasite from animals to human beings [5].Protozoan parasites are responsible for causing severe infections both in humans and animals worldwide [6].The impact of diseases caused by these organisms on health and productivity of farm animals and human beings is huge, though a fair economic assessment on these aspect is yet to be worked out from India.
Among various protozoan diseases haemoprotozoan disease play a crucial effect in declining the production by the animals. Trypanosomosis is a haemoprotozoan disease caused by various members of Trypanosoma spp. and is transmitted through the biting vector flies wherein the parasite may undergo biological or mechanical transmission. Various Trypanosoma spp. infect a wide variety of domestic and wild animals like horses, mule, donkey, camel, cattle, buffaloes, sheep, goat, dogs, pig, elephant, deer, foxes, tiger and jackals with major clinical signs of high intermittent fever, anaemia, loss of weight, edema of dependent parts, nervous symptoms and abortion is responsible for major production losses.
Animal trypanosomosis is now a days considered as a permanent constraint for livestock productivity in Africa, Asia and Latin America with their geographical distribution still evolving [7]. Trypanosoma evansi (Trypanozoon) was the first pathogenic mammalian trypanosome discovered by Griffith Evans in 1980, from the blood of Indian camel and later from the blood of Indian equines [8]. T. evansi is thought to be derived from T. bruceii (a cyclically transmitted trypanosome by tsetse flies), but parasite is no longer able to undergo its biological cycle in Glossina spp. fly because of the loss of the maxicircles of kinetoplastic mitochondrial DNA [9-11]. In the Indian sub-continent, the disease is mainly endemic and most of the epizootics have occurred particularly in bovines with a high mortality rate ranging from 20-90% [12,13]. It is thought to produce immunosuppression resulting in concurrent infection and poor immune response to vaccines [14,15].
Human trypanosomosis is endemic in Africa and South America. In Africa, the disease, known as human African trypanosomosis (HAT) or sleeping sickness, is caused by Trypanosoma brucei gambiense (chronic form) or T. b. rhodesiense (acute form), whereas the American trypanosomosis, known as Chagas’ disease, is caused by T. cruzi. Sleeping sickness and Chagas’ disease are both transmitted by vectors [8]. In addition to human infectious trypanosomes, a variety of other species cause animal trypanosomosis with a wide geographic distribution. Nagana is caused by T. b. brucei in Africa and affects cattle; T. congolense and T. vivax infect domestic and small animals; and surra is caused by T. (Trypanozoon) evansi and infects mainly camels, cattle, and buffalos and other wild animals on all continents [8].
Human infection by animal species of trypanosomes is not seen due to presence of trypanolytic factor in human serum [5]. But as the parasites continuously seems to change its geographical distribution, host specificity, drug resistant; these factors make this disease entity an upcoming emering threat to human society and also raising alarming debatable one health issue to public health experts and parasitologists. Here, in this review we will discuss about the facts and features of previously reported cases of atypical human trypanosomosis and some more burning issues regarding research need to explore this disease entity.
Humans are resistant to infection with T. evansi and other related African trypanosomes except Trypanosoma brucei rhodesiense and T. bruceii gambiense because serum resistance-associated protein (SRA) gene is absent in this group of trypanosomes, which interacts specifically with APOL1 and neutralizes it. Due to trypanolytic activity of human plasma T. evansi has never been considered capable of infecting humans [16].
Cases of atypical human trypanosomosis from Indian sub continent
A total 20 humans cases have been reported, including nine T. lewisi, (six from India, one each from Gambia, Thialand, Malaysia), 5 T. evansi (3 from India, 1 each from Srilanka & Egypt), 4 T. bruceii (1 each from Ghana, France, Congo, Ethopia), 1 T. vivax (Ghana) & 1 T. congolense Coˆ te d’Ivoire (Figure 1).
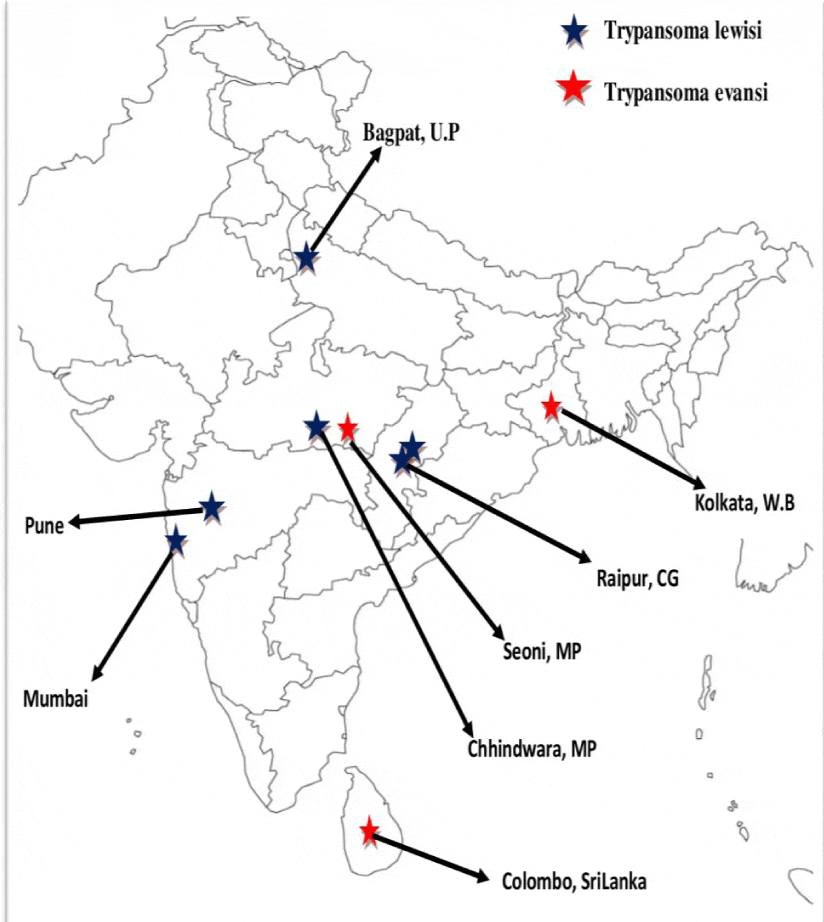
A serological surveys conducted from India, China, Somalia indicated an exposed population from 2.5 to 4% for T. evansi or T. lewisi antigens [17]. Total number ten cases of atypical human trypanosomosis had been reported from Indian sub-continent. Out of ten cases these, nine cases were from India and one from Sri Lanka (Tables 1,2). During the last few years, three cases of human T. evansi have been reported from the Indian subcontinent (one from Sri Lanka and two from India). Four more atypical human cases of trypanosomosis caused by rat trypanosome, T. lewisi have been reported. Moreover, two casualties from India due to non-tsetse transmitted trypanosomosis (NTTT) were also reported [16,17]. Due to these facts in India, animal trypanosomosis seems to be a matter of concern for public health. In summer 1999, in Colombo a patient of trypanosomosis with the symptoms of headache and numerous trypanosomes in his blood was reported. Occurrence of hyperthermia coincided with increased number of trypanosomes in blood. Frequent contacts with cattle, were suspected to be T. evansi infection in patient. In February 2003, without any treatment prescribed the patient was thought to be a case of self-healing, although extremely weak after undergoing many phases of exacerbation and effervescence of the disease [18].
Out of the twenty atypical human trypanosomosis cases (nine by T. lewisi, five by T. evansi, four by T. bruceii, one each by T. vivax and T. congolense), 6 are from infants and 9 are from India. Two more cases have very recently been detected in Puducherry [19]. Particularly in the State of Maharashtra the concern is more serious because one case of T. evansi and two cases of T. lewisi have been reported in a span of 3 years.
In West Bengal (1903) the 1st atypical human trypanosomosis was reported from a 40 year old female suffering from headache, fever and died within two days. The blood examination revealed Trypanosoma spp. and the area was prevalent only for the T. evansi. The prevalence of this parasite is 8-10% in cattle and buffaloes with seasonal variation reaching a peak of 30% in monsoon [18]. Keeping aforementioned facts and absence of proper diagnostic aids, it was presumed that the Trypanosoma spp. which was observed in the patient was T. evansi.
In India the first parasitologically and immuno-molecularly confirmed atypical case of human trypanosomosis caused by T. evansi, was reported in September 2004 from a 45 years old male herdsman staying in village Shivani block Sindewahi in Chandrapur district of Maharashtra. The village has a population of about 3000 and is very close to the Tadoba Reserve forest. The herdsman suffered episodic fever associated with sensory disturbances and violent behavior. Blood smear examination revealed presence of approximately 106 trypanosomes per milliliter of blood [5]. On the basis of parasitologic, immunologic, molecular diagnostic tests (PCR i.e. polymerase chain reaction) and genetic characterization, the parasite was identified as T. evansi [20]. After treatment with suramin (Virbac) intravenously @ 20 mg/kg weekly [5], patient recovered completely in 6 months of initial infection. A series of investigations followed to ascertain the factors responsible for the occurrence of this unique case of T. evansi and the explanation for this unusual infection was related to the patient, whose serum was found to have no trypanolytic activity due to absence of APOL1, which was linked to frameshift mutation in both APOL1 alleles [21].
Atypical T. lewisi infection was first reported from adult couple hailing from a rat-infested rural area from Raipur, Madhya Pradesh in 1974. Patients were suffering from fever and malaise and were found to have heavy T. lewisi parasitaemia [22]. Moreover again in September 2006, a recent case of atypical human trypanosomosis in a man aged 57 years, residing in a small village in district Pune (Maharashtra) has been reported. He also suffered from chronic intermittent fever, anemia, hepatosplenomegaly and edema on feet [23]. The investigations in March 2007 revealed that it was a case of T. lewisi. No other cases were reported after primary epidemiological investigations in the village. The epidemiological investigations indicated the possibility of transmission was through rat fleas. Of the 8 rats trapped from the vicinity of the house, two were found positive for T. lewisi by PCR [23]. Host switching of T. lewisi from their natural rodent hosts to humans might have occurred due to co-habitation of humans and rats in human dwellings both in urban and rural settings and human exposure to infected fleas [24]. Preliminary investigation before treatment revealed hemoglobin (Hb) 8.7 g., white blood cell (WBC) 3,000, platelets 135,000, serum creatinine 0.7, serum bilirubin 0.5, SGPT 11, alkaline phosphtase 273, apolipoprotein A154.06 mg/dl. Magnetic resonance imaging (MRI) revealed signs of cortical atrophy, ultrasonography showed splenomegaly [25]. After 1st dose of drug the values of various haematological and biochemical were were: hemoglobin 7.7 g, white blood cell count 2800, platelets 47,000, serum creatinine 0.8, serum bilirubin 0.8, SGPT 96, alkaline phoshphatase 78, urine albumin positive. Bone marrow suppression may have resulted in moderate anemia and thrombocytopenia [25]. After 2nd dose of the drug the haematobiochemical levels were Hb 9.3 g, WBC 4600, platelets 184,000, serum bilirubin 0.6, SGPT 32, alkaline phoshphatase 86, serum creratinine -0.9, serum protein 7.8, serum albumin 3.1 [25]. During treatment the patient expired after second dose of Suramin on 21 June 2007. After Postmortem, parasites were seen in the pericardial and ascitic fluid but the cerebrospinal fluid (CSF) did not show trypanosomes. The genetic study for mutation in the APOL 1 gene revealed that the four DNA fragments from the patient did not have any mutation in the amplified exonic fragments [25].
A case was reported on 1 September 2006, with history of fever since 5 days. The patient was one and a half month old girl born of non-consanguineous marriage from Andheri, Mumbai. She was febrile and had hepatosplenomegaly. Large size of kinetoblast, pointed posterior end of cell and lack of undulating membrane were suggestive of trypanosomes (T. lewisi) in the peripheral blood smear. Haematobiochemical parameters were also altered [26, 27].
In 2014 at Nagpur, Maharashtra, an adult male livestock farmer was presented with history of febrile episodes since 12 months. Anemia, firm splenomegaly and edema on feet were reported on examination. He received the second dose of intravenous Suramin (for T. lewisi) in the intensive care unit (ICU) under strict medical supervision. The patient was symptomatically better following this therapy and was thus discharged with regular follow-up advice [28].
Animal trypanosomosis can infect humans given the right combination of environmental, host related and organism-related factors and these patients should be managed diligently.
APOL1: A future hope for treatment of Trypanosoma evansi infection
Human innate immunity against Trypanosoma brucei brucei is due to the trypanolytic activity of a human-specific apolipoprotein bound to high-density lipoproteins, known as apolipoprotein L1 (APOL1) [29]. APOL1 is absorbed by the parasite by endocytosis and triggers the formation of anion selective pores in the lysosomal membrane, which induces uncontrolled osmotic swelling of this compartment and subsequently cell death [21,30,31]. The trypanolytic activity of proteins as APOL1 and therapies involving human blood for the control of T. bruceii and T. evansi infections have been described [16,32]. Researchers investigated the absence of this protein in the serum of an infected farmer who had high parasitemia and clinical signs of trypanosomosis. The results indicated an absence of APOL1, which is responsible for the trypanolytic activity seen in the human serum [21].
Studies revealed that trypanosome isolates belonging to the Trypanozoon subgroup were sensitive to therapy using human plasma [21,33]. Recently, a research group found that mice infected with T. evansi when treated with human plasma and blood can eliminate the parasite from circulation [34].
Trypanosoma brucei and T. evansi are both sensitive to treatment with human plasma [21,34]. Researchers have used plasma therapy from different hosts to treat trypanosomosis [35,36]. Studies have shown that rabbits are very resistant to T. evansi infection [37-39], but this resistance is not yet clear. It is probably associated with proteins with trypanocidal activity found in the plasma of rabbits as well as APOL1 in humans [30]. Studies have reported that haptoglobin protein and apolipoprotein A1 (APOA1) are also components of the two trypanolytic factors in human serum [40].
A research group has investigated in recent years alternative treatments for T. evansi infection because chemotherapy with diminazene aceturate and suramim used in the treatment of trypanosomosis has proved ineffective in many cases [24,41,42]. Studies have demonstrated that these drugs have limited effectiveness because they do not cross the blood-brain barrier, thereby creating a potential refuge for trypanosomes during the systemic phase of drug action [43,44]. Therefore, research with herbal medicines, immunotherapy and new chemical compounds are needed to fight T. evansi [24].
Although emerging human trypanosomosis in India is not the present important problem. But the existence of problem, without linkage to the disease transmission from endemic area, leads the consideration on the possibility of cross species zoonosis from locally infected animals. Unhygienic animal husbandary and veterinary practices, faulty disease diagnosis, poor epidemiological survey, lack of knowledge about disease transmission, vector habitat, drug resistance, increase in transporation, no standard quarantine measure, abrupt environmental changes are some factors which may lead to human trypanosomosis as a emerging threat in India.
Conclusion and Recommendation
There are so many hurdles and lacunae in surveillance and diagnosis of atypical human trypanosomosis. Now, there is urgent need of hour to develop and standardize laboratories country wide for molecular studies required for a species-specific diagnosis, presently which are very limited in India. Few protocols for research, diagnosis and development can be adopted. Firstly, large scale sero-surveillance throughout length and width of Indian subcontinent is required to establish a case management protocol of detected cases. Secondly, Training of malaria technicians, veterinarian and veterinary assistant staff is necessary who can examine millions of blood smears to also look for trypanosomes, is an important strategy to find out hidden cases. Thirdly, there is need to establish a collaborative network for understanding, studying and research related to atypical human trypanosomosis involving Food and Agricultural Organization (FAO), World Animal Health Organization (WAHO/OIE Organization Internationale des Epizooties) and World Health Organization (WHO) at international level. At regional level medical institution and veterinary institution professionals can also work on this one health issue task in collaborative manner. So, it is critical and also crucial to say that animal origin trypanosome may have potential to invade human health defence system, so this one health issue should be taken seriously. Furthermore epidemiological surveillance in the field and advance laboratories are warranted. Livestock and animal husbandry practices should be deal with utmost care to minimize any man made accidental infection.
- Mahima AK, Verma A, Kumar A, Rahal, Kumar V (2012) Veterinarian for sustainable development of humanity. Asian J Anim. Vet 7: 752-753.
- Dash AP, Bhatia R, Sunyoto T, Mourya DT (2013) Emerging and re-emerging arboviral diseases in Southeast Asia. J Vector Borne Dis 50: 77-84. Link: https://goo.gl/fZkWko
- Horby PW, Pfeiffer D, Oshitani H (2013) Prospects for emerging infections in East and southeast Asia 10 years after severe acute respiratory syndrome. Emerg Infect Dis 19: 853-60. Link: https://goo.gl/eE9BZl
- Gongal G (2013) One Health approach in the South East Asia region: opportunities and challenges. Curr Top Microbiol Immunol 366:113-122. Link: https://goo.gl/Bl9YsX
- Joshi PP, Shegokar VR, Powar RM, Herder S, Katti R, et al. (2005) Human trypanosomosis caused by Trypanosoma evansi in India: The first case report. Am J Trop Med Hyg 73: 491–495. Link: https://goo.gl/zLbqWW
- Salih DA, El Hussein AM, Singla LD (2015) Diagnostic approaches for tick borne haemoparasitic diseases in livestock. J Vet Med Anim Health 7: 45-56. Link: https://goo.gl/f0hZSs
- Desquesnes M, Dargantes A, Lai DH, Lun P, Holzmuller, et al. (2013a) Trypanosoma evansi and surra: A review and perspectives on transmission, epidemiology and control, impact and zoonotic aspects. BioMed Res Int 2013: 321237. Link: https://goo.gl/RMzQUG
- Hoare CA (1972) The Trypanosomes of Mammals. A Zoological Monograph. Oxford, United Kingdom: Blackwell Scientific Publications, Oxford, UK, 749. Link: https://goo.gl/zdxs6k
- Borst P, Fase-Fowler F, Gibson WC (1987) Kinetoplast DNA of Trypanosoma evansi. Mol. Biochem. Parasitol 23: 31-38. Link: https://goo.gl/CR8pVJ
- Lun ZR, Desser SS (1995) Is the broad range of hosts and geographical distribution of Trypanosoma evansi attributable to the loss of maxicircle kinetoplast DNA? Parasitol. Today 11: 131-133. Link: https://goo.gl/86V609
- Lai DH, Hashimi H, Lun ZR, Ayala FJ, Lukes J (2008) Adaptations of Trypanosoma brucei to gradual loss of kinetoplast DNA: Trypanosoma equiperdum and Trypanosoma evansi are petite mutants of T. bruceii . Proc Nat Acad Sci 105: 1999-2004. Link: https://goo.gl/9idbry
- Gill BS (1977) Trypanosomes and Trypanosomosis of Indian Livestock. ICAR Publication, Pusa, New Delhi. Link: https://goo.gl/Fv7olG
- Juyal PD, Singla LD, Kaur P (2005) Management of surra due to Trypanosoma evansi in India: an overview. In: Infectious Diseases of Domestic Animals and Zoonosis in India, Tandon V and Dhawan BN (Eds), Proceedings of the National Academy of Sciences India Section B: Biological Science 75 (Special issue): 109-120.
- Gupta MP, Kumar H, Singla LD (2009) Trypanosomosis concurrent to tuberculosis in black bucks. Indian Vet J 86: 727-728. Link: https://goo.gl/agJDUA
- Singla LD, Juyal PD, Sharma NS (2009) Immune responses to haemorrhagic septicaemia (HS) vaccination in Trypanosoma evansi infected buffalo-calves. Trop. Anim. Health Prod 42: 589-595. Link: https://goo.gl/FRzMoH
- Juyal PD, Singla LD, Saxena HM (1998) In vivo activity of human serum against T. evansi infection in Swiss albino mice. J Parasitic Dis 22: z7-68.
- Truc P (2010) Touratier Tuding atypical human trypanosomosis: towards an international network initiative. Annual Report of O.I.E., Bordeaux, France, Annexure X.
- Truc P, Büscher P, Cuny G, Gonzatti MI, Jannin J, et al. (2013) Atypical Human Infections by Animal Trypanosomes. PLOS Neg Trop Dis 7: e2256 Link: https://goo.gl/oUyCtO
- Singla LD, Sumbria D (2016) Human Atypical Trypanosomosis in Indian Subcontinent. Veterinaria 4: 7-10. Link: https://goo.gl/AGA063
- True P, Gibson W, Herder S (2007) Genetic characterization of Trypanosoma evansi isolated from a human patient in India. Inf. Gen. Evol 7: 305-307. https://goo.gl/Zc53Ku
- Vanhollebeke B, True P, Poelvoorde P, Pays A, Joshi PP, et al. (2006) Human Trypanosoma evansi infection linked to a lack of Apolipoprotein L-I. N. Engl J Med 355: 2752-2756. Link: https://goo.gl/fgzR3a
- Shrivastava KK, Shrivastava GP (1974) Two cases of Trypanosoma (Herpetosoma) species infection of man in India. Trans R Soc Trop Med Hyg 68: 143-144. Link: https://goo.gl/8FRvWB
- Banerjee PS, Basavaraj A, Kaur R, Rana UVS, Tewari AK, et al. (2008) Fatal case of Trypanosoma lewisi in a human patient in India. 40h Asia-Pacific Academic Consortium for Public Health Conference, Vietnam, 4-6.
- Silva AS, Andrade-Neto OAS, Costa MM, Wolkmer P, Mazzantti CM, et al. (2010) Tripanossomose em equinos na região sul do Brasil. Acta Sci Vet 38: 113-120. Link: https://goo.gl/d3kQFg
- Doke PP, Kar A (2011) A fatal case of Trypanosoma lewesi in Maharashtra, India. Ann of Trop Med Public Health 4: 91-95. Link: https://goo.gl/K7wgtK
- Shah I, Uma S. Ali US, Andankar P, Joshi RR (2011) Trypanosomosis in an infant from India. J Vector Borne Dis 48: 122–123. Link: https://goo.gl/Bql9Be
- Shah MAA, Rehman K, Rehman F, He N (2013) Present status of camel Trypnosomiasis in Pakistan, a review of literature. Sci Lett 1: 30-33. Link: https://goo.gl/5eyweY
- Warpe BM, More SV (2014) A rare Indian case of human trypanosomosis caused by Trypanosoma lewisi-like parasites. J Biosci Tech 5: 564-567. Link: https://goo.gl/VuXRjd
- Vanhamme L1, Paturiaux-Hanocq F, Poelvoorde P, Nolan DP, Lins L, et al. (2003) Apolipoprotein L-I is the trypanosome lytic factor of human serum. Nature 422(6927): 83-87. Link: https://goo.gl/YDK8OQ
- Pérez-Morga D, Vanhollebeke B, Paturiaux-Hanocq F, Nolan DP, Lins L, Homblé F (2005) Apolipoprotein L-I promotes trypanosome lysis by forming pores in lysosomal membranes. Sci 309: 469-472. Link: https://goo.gl/OdA3J9
- Pays E, Vanhollebeke B, Vanhamme L, Paturiaux-Hanocq F, Nolan DP, et al. (2006) The trypanolytic factor of human serum. Nat Rev Microbiol 4: 477-486. Link: https://goo.gl/zFHoHF
- Pays E, Vanhollebeke B (2008) Mutual self-defence: the trypanolytic factor story. Microb infect. 10: 985-989. Link: https://goo.gl/P6SejD
- Hawking F (1978) The resistance of Trypanosoma congolense, T. vivax and T. evansi to human plasma. Trans R Soc Trop Med Hyg 72(4): 405‑407. Link: https://goo.gl/AG018V
- Otto MA, Da Silva AS, Gressler LT, Farret MH, Tavares KC, et al. (2010) Susceptibility of Trypanosoma evansi to human blood and plasma in infected mice. Vet Parasitol 168: 1-4. Link: https://goo.gl/nQhO8R
- Wechsler DS, Kongshavn PA (1988) Further characterization of the curative antibodies in Trypanosoma musculi infection. Infect Immunol 56: 2379-2384. Link: https://goo.gl/MZc6qe
- Otto MA, Faccio L, Soares JF, Soares CDM, Gressler LT, et al. ( 2009) Plasma de coelhos no controle da infecção por Trypanosoma evansi em ratos. Vet Zootec 16: 379-384. Link: https://goo.gl/yXBB3o
- Uche UE, Jones TW, Boid R (1992) Antibody patterns in rabbits showing different levels of susceptibility to an experimental Trypanosoma evansi infection. Acta Trop 52: 139-147. Link: https://goo.gl/t94YWF
- Silva AS, Costa MM, Cargnelutti JF, Lopes STA, Monteiro SG (2007) Alterações bioquímicas em coelhos infectados experimentalmente pelo Trypanosoma evansi. Rev Bras Parasitol Vet 16: 43-46. Link: https://goo.gl/hW8tWv
- Silva AS, Costa MM, Cargnelutti JF, Lopes STA, Monteiro SG (2008a) Alterações hematológicas em coelhos infectados experimentalmente pelo Trypanosoma evansi. Ciênc Rural 38: 538-542. Link: https://goo.gl/DD4qUH
- Tomlinson S, Muranjan M, Nussenzweig V, Raper J (1997) Haptoglobin-related protein and apolipoprotein AI are components of the two trypanolytic factors in human serum. Mol Biochem Parasitol 86: 117-120. Link: https://goo.gl/J3KAVI
- Tuntasuvan D, Jarabrum W, Viseshakul N, Mohkaew K, Borisutsuwan S, et al. (2003) Chemotherapy of surra in horses and mules with diminazene aceturate. Vet Parasitol 110: 227-233. Link: https://goo.gl/8N9GPR
- Silva AS, Tochetto C, Zanette RA, Pierezan F, Rissi DR, et al. (2008b) Aceturato de diminazeno e dipropionato de imidocarb no controle de infecção por Trypanosoma evansi em Rattus norvegicus infectados experimentalmente. Cienc Rural 38: 1357-1362. Link: https://goo.gl/OdSjfF
- Jennings FW, Whitelaw DD, Urquhart GM (1977) The relationship between duration of infection with Trypanosoma brucei in mice and the efficacy of chemotherapy. Vet. Parasitol 75: 145-153. Link: https://goo.gl/58gve5
- Spinosa HS, Górniak SL, Bernardi MM (1999) Farmacologia aplicada à medicina veterinária. Rio de Janeiro: Guanabara koogan.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
