International Journal of Veterinary Science and Research
Factors Affecting Lethality of Bisphenol a on Biomphalaria alexandrina Snails
Sara A Mansour1,2, Maha FM Soliman1*, Fatma AA El Deeb2 and Nahla S El-Shenawy1
2Environmental Research Department, Theodor Bilharz Research Institute, Giza, Egypt
Cite this as
Mansour SA, Soliman MFM, El Deeb FAA, El-Shenawy NS (2016) Factors Affecting Lethality of Bisphenol a on Biomphalaria alexandrina Snails. Int J Vet Sci Res 2(1): 007-013. DOI: 10.17352/ijvsr.000007The present study was undertaken to investigate the influence of some environmental factors including temperature, water vegetation, bed mud and pH on LC50, LC90and LT50 of bisphenol A (BPA) on the snail host of Schistosoma mansoni, Biomphalaria alexandrina. Effects of exposure to the sublethal concentrations of BPA on some biological aspects of the snails and on the cercarial output form S. mansoni infected snails were studied. Results showed that temperature, water vegetation, bed mud and pH markedly affected the lethality of BPA. The biological parameters of B. alexandrina including survival rate, egg hatchability and egg laying capacity were greatly affected by exposure to BPA and the response was dose dependent. Regarding the possible effect of BPA on transmission of schistosomiasis, results showed that exposure to different concentration of BPA for 7 days before miracidial infection caused the death of all the snails before reaching the patent period while, no cercarial output was recorded from snails exposed to BPA for 24 hrs till their death. In conclusion, our results showed that the environmental characteristics may alter the biological impacts of BPA and the exposure of snail to BPA may affect the transmission of schistosomiasis.
Introduction
Studying the environmental conditions related to the occurrence of snail intermediate host can provide valuable information on its breeding sites and consequently on the model of schistosomiasis transmission [1]. The anthropogenic ecological transformations have a considerable impact on altering many of the environmental and social conditions necessary for schistosomiasis transmission [2].Human activities resulting in chemical contamination of the environment have increased the potential stresses on molluscs in exposed habitats [3].
Bisphenol a (BPA) is one of the environmental contaminants widely used in the manufacture of polycarbonate plastic (e.g., water bottles), epoxy resins (e.g., inside coating in metallic food cans) and as a non–polymer additive to other plastics [4,5]. BPA is a pseudo-persistent chemical, which despite its short half-life is ubiquitous in the environment because of continuous release [6]. Post-consumer releases are primarily via effluent discharge from municipal wastewater treatment plants, leaching from landfills, combustion of domestic waste, and the natural breakdown of plastics in the environment [7,8].
It has been suggested that some invertebrates appear to be quite sensitive to BPA, and effects have been documented at environmentally relevant concentrations [6]. Both midge Chironomus riparius larvae and the marine copepod Tigriopus japonicus showed developmental inhibition at very low concentrations of BPA [9,10]. However, higher exposure caused premature larval metamorphosis and settlement in the marine polychaete worm Capitella capitata [11].
In the freshwater snail Marisa cornuarietis, exposure levels >1.0 mg L-1 were found to result in super-feminization (additional female organs, enlarged sex glands, oviduct deformities, and increased fecundity), oviduct rupture, and mortality [12]. In the mollusk Mytilus edulis, spawning induction, as well as oocyte and ovarian follicle damage, was observed following BPA exposure for 3 weeks at 50.0 mg L-1 [13]. The effect of BPA appears to vary considerably among related taxa, and it appears that some invertebrates may be hypersensitive to BPA exposure (freshwater molluscs and insect larvae, and marine copepods in particular).
The present study was undertaken to investigate the influence of some environmental factors including temperature, water vegetation, bed mud and pH on the effect of bisphenol A on the fresh water snails, B. alexandrina and consequently the transmission of schistosomiasis. The possible effect of BPA on the Schistosoma cercarial output form the snails was also studied.
Material and Methods
Maintenance and rearing of B. alexandrina snails
B. alexandrina snails were collected from fresh water canals in Warrak El-Arab village, Giza Governorate. The collected snails were maintained in glass tank filled with dechlorinated tap water under constant temperature (25 ± 2 ºC) with diurnal alteration and fed on dried lettuce leaves for at least three weeks before being used for the following experiments. Maintenance and rearing of the snails were done according to [14].
Determination of LC50, LC90and LT50 of bisphenol A against B. alexandrina snails
Bisphenol A (4,4´–isopropylidinedi-phenol) was purchased from Sigma Aldrich Company, Germany. Stock solution of BPA (100 mg L-1) was prepared according to the method of [15]. A series of concentrations (1.0, 3.0, 5.0, 7.0, 9.0, 11.0 and 13.0 mg L-1) were used for determination the LC50 and LC90values of BPA. Three replicates of adult snails (10 snails/replicate/L capacity tank) with 9.0 - 11.0 mm shell diameter were used for each concentration after three weeks of maintenance. Snails were exposed for 24 hrs to BPA concentrations then transferred to dechlorinated tap water for another 24 hrs for recovery. Mortality rate was determined after recovery period [16]. A set of control snails was prepared using dechlorinated water was run parallel to tested concentrations. Computation of LC50 and LC90values and slope function were determined utilizing the statistical program SPSS (2001) for windows.
The LT50 was carried out by exposing separate groups of snails to the previous concentrations of BPA for 24, 48, 72 and 96 hrs, followed by 24 hrs of recovery for each exposure period.
Studying the effect of certain environmental factors on LC50 and LC90values of BPA
This experiment was designed to evaluate the effect of temperature, water vegetation, river- bed mud and pH on LC50 values of BPA. Three replicates of adult snails (10 snails / replica) were used for each experiment. The control group of snails was maintained at the same experimental condition in de-chlorinated tap water. Exposure and recovery periods for all experimental tests were 24 hrs. Mortality rates were determined in each experiment after recovery period in clean de-chlorinated water for 24 hrs [17].
Temperature: snails were exposed to LC50 and LC90(9.7 and 12.3 mg L-1, respectively) of BPA at 18, 24 and 30 ºC.
Water vegetation: water plants namely Lemna gibba (Family: Lemnaceae) and Ceratophyllum demersum (Family: Ceratophyllaceae) were used. Snails were exposed to LC50 and LC90with / without L. Gibba or C. demersum in different amount (1.0, 2.0 and 4.0 g L-1).
River-Bed mud particles: snails were deposed to different amounts of very fine mud (5.0 and 10.0 g L-1) with LC50 and LC90of BPA. A set of control snails was prepared using de-chlorinated water and mud at the same amount. Tanks were provided with gentle air stream to maintain continuous and thoroughly mixing the mud [18].
pH: LC50 and LC90of BPA were prepared in standard reference water solutions previously adjusted with NaOH or HCl at pH values of 9.0, 7.0 and 4.0. Three replicates were performed for each pH value and BPA concentration. Control snails were prepared in de-chlorinated water having the same experimental pH values [17]. The pH measurements were made only once in this experiment, before adding BPA.
Effect of sub-lethal concentrations of BPA on some biological parameters of the snail
The semi-static system was used with renewing the treatment solution twice a week to study the effect of sublethal concentrations (0.01, 0.05 and 0.1 of LC50-24 hrs) of BPA on some biological parameters of B. alexandrina for 28 days. Half-life of BPA from manufacturing effluent is 2.5:4.0 days [19].
Survival rate and shell diameter: Juvenile snails (4.0 ± 0.5 mm) were used in this experiment. For each concentration, a group of 30 juvenile snails (3 replicates) was exposed in glass jar of 1000 mL capacity. Another group of snails was maintained in dechlorinated tap water as control. Snails were fed daily with dried lettuce leaves. The survival rate was recorded weekly [20]. The shell diameter was measured weekly using a caliper. The shell diameter of juvenile snails was calculated as the difference between size of snails of each week and the size of snails in the previous week divided by the number of lived snails in this week according to the method of [21].
Hatchability rate of the eggs: The aged egg masses of one and six days were collected from nylon sheets placed on the water surface in the aquaria contained B. alexandrina snails that were supplied with dried lettuce leaves and tetramine (fish food) for being able of oviposition. Three replicates of each egg masses age were used separately. Each egg-age was exposed to the sublethal concentration of BPA. A group of each egg-age was maintained in dechlorinated water as control. Then, all treatment and control egg masses were transferred to clean dechlorinated water to recover for 24 hrs at 25.0 ±1.0 ºC [22].
Egg masses were examined daily during the experimental period under a stereomicroscope and the newly hatched snails were recorded. At the end of experiment, the percentage of hatchability was calculated by dividing the number of newly hatched snails by total number of eggs at the beginning of experiment [23].
Egg-laying capacity: Thirty adult snails (9.0-11.0 mm) were used into three replicates for each experimental concentration. Untreated normal snails were maintained under the same experimental conditions and at 25.0 ± 2.0 ºC. Daily dried lettuce leaves food was added. White foams were putted to aquaria for egg deposition. The egg masses laid by exposed and control snails were collected and counted weekly.
Dead snails were removed from aquaria and the number of lived snails at the end of each week was recorded. Snail’s egg clutches deposited on the foam and the wall of the aquaria were gently collected weekly and their egg content was counted using a dissecting microscope. The surviving snails in each aquarium counted also weekly [24]. The terms used to express the tested parameters, (Lx) that refers to the survivorship or the ratio of surviving snails in each week, (Mx) represents the egg laying capacity or mean number of eggs/snail/week. (LxMx) refers to the reproductive rate in each week while (∑LxMx) is the total reproductive rate at the end of the experiment.
Experimental infection of B. alexandrina snails with Schistosoma mansoni
Schistosoma mansoni ova were obtained from Schistosomiasis Biological Supply Center (SPSC), Theodor Bilharzia Research Institute (TBRI), and Giza, Egypt. They were left in clean dechlorinated water for hatching under a desk lamp then fresh hatch miracidia were used in bio-assay and infection tests. Two replicates of adult B. alexandrina snails (10snails/replicate in 1Lglass container) were exposed to sublethal concentrations of BPA either for 24 hrs or for 7 days before miracidial exposure. Snails were exposed to miracidia individually (10 fresh hatched miracidia/snail) for 24 h under ceiling illumination. After that, snails were transferred to clean de-chlorinated water (26 ± 2°C) and daily fed with oven dried lettuce leaves throughout the pre-patent and patent periods [25]. A control group of two replicates was exposed to miracidia concurrently with the experimental snails and treated similarly. Dead snails were removed daily and surviving snails were individually examined once weekly for cercarial shedding.
Statistical analysis
The hatchability percent and survival rate were analyzed by Chi-square values of contingency tables [26]. The data of growth rate was statistically analyzed for the significance difference between control and treated groups by using T-test and values were expressed as means ± S.E.
Results
After 34 hrs of exposure, there was no mortality recorded in the 0.0 to 5.0 mg L-1 concentrations. Mortality was 15.0 % for 7.0 mg L-1 and increased to be 35.0 % and 60 % for 9.0 and 11.0 mg L-1, respectively (Table 1). Mortality was recorded 100 % when 13.0 mg L-1 concentration was applied. The calculated lethal concentrations LC50-24 hrs and LC90-24 hrs were 9.9 and 12.8 mg L-1, respectively.
The toxicity of BPA against B. alexandrina was time and dose-dependent. There was a significant correlation between all LC50 values and exposure periods (Table 1). The LC50 values decreased from 9.9 mg L-1 at 24 hrs to 1.5 mg L-1 at 96 hrs as well as the LC90values decreased from 12.8 to 4.2 mg L-1 at 24 and 96 hrs, respectively.
Time required reaching the values of LT50 and LT90 was reversely proportional to concentrations. The LT50 values were 69.4, 49.8, 32.9 and 19.3 hrs for the concentrations 5.0, 7.0, 9.0, 11 and 13, respectively. LT90 values decreased from 94.2 to 45.9 hrs by increasing the exposure concentration to the snails from 5.0 to11.0 mg L-1, respectively.
Data in Table 2 showed that the percentage of dead snails increased by increasing temperature. BPA cause no mortality observation for LC50 value at 18 °C while, 13 % of snails died when LC90was applied. Mortality rate was much higher at 24 ºC than that 18 °C. The mortality percentages were 50 % and 80 % in snails that exposed to LC50 and LC90values of BPA at 24 ºC, respectively. However, the temperature of 30 ºC greatly increased the snails’ mortality to be 80% and 100% at concentrations of LC50 and LC90of BPA (Table 2).
Table 2 showed that the effect of different densities of L.gibba and C. demersum on the mortality of B. alexandrina exposed to LC50 and LC90of BPA. For both plant, the LC50 of BPA that mixed with different amount of plant densities (1.0, 2.0 and 4.0 g L-1) had more toxicity and caused more mortality than applied LC50 value without plant. On the other hand, results showed that both plants have no effect on the LC90values at 1.0 and 2.0 g of both densities. However, the mortality was slightly increased at LC90value of BPA with 4.0 g of C. demersum when compared with amount of L. Gibba in which all the snails died.
Mud particles influenced the BPA toxicity against B. alexandrina snails as shown in Table 3. The values of LC50 and LC90of BPA against snails were decreased when mixed with 5.0 g L-1 of mud to reach to 30% and 66.66%, respectively. The percentage of the snails died when exposed to LC50 of BPA (9.7 mg L-1) mixed with 10.0 g L-1 of mud was 70 %. The same pattern was recorded for LC90of BPA that mixed with 10.0 g L-1 mud, the percentage of dead animals was 76.6 % (Table 2).
Toxicity of BPA against B. alexandrina differed by variation in the pH values of water (Table 2). In alkaline medium at pH 9.0, the percentage of dead snails was 43.3%. However, the percentage of dead snails sharply decreased in to reach to 23.3 % at acidic medium (pH 4.0). The LC90of BPA against snails also decreased to record 76.6 and 66.66 % of died snails in alkaline and acidic media, respectively (Table 2).
Regarding the effect of BPA toxicity on juvenile snails, the results in Table 3 indicated that the survival rate (Lx) of treated snails with 0.01- and 0.05- LC50 for 24 hrs (0.097 and 0.485 mg L-1, respectively) showed a gradual decrease during the experiments. On the contrary, the Lx value of juveniles exposed to 0.97 mg L-1 was markedly decreased at the third week that was significant at as compared to 100 % Lx in control group. Comparing the mean shell diameter of control group with that of treated ones, results did not show any significant differences (Table 3).
Table 4 showed the effect of BPA toxicity on hatchability rate of aged egg masses of one and six days old. There was slightly decrease in hatchability rate when 1-day-aged eggs were exposed to 0.097 and 0.485 mg L-1 of BPA for one day. However, the hatchability was decreased at 0.97 mg L-1 to reach to 72.4 % after one day of exposure. The continuous exposure the egg to BPA for a week, the hatchability rate of one- eggs and six-egg old showed gradual decrease with the three tested concentrations but eggs of six days old were less susceptible than one day old when exposed to 0.97 mg L-1 of BPA either for a day or a week.
Lx of adult snails was slightly decreased after exposure to 0.097 mg L-1 of BPA for two and three weeks as compared with control (Table 5). The Lx of snails exposed to 0.485 and 0.97 mg L-1 was decreased gradually until the 3rd week then had a very highly significant decrease by the 4th week. Comparing the fecundity (Mx) and reproductive rate (LxMx) of snails treated with 0.097 mg L-1, data revealed that the two parameters were affected markedly by BPA exposure. The Mx and LxMx values were higher than those of control after four weeks of exposure to low concentration of BPA. On the other hand, the Mx and LxMx were lower as compared to control group during the whole experimental period in the groups that exposed to 0.485 and 0.97 mg L-1. There was a reduction in the total LxMx in snails reaching to 87.2 and 77.9 as the effect of 0.485 and 0.97 mg L-1, respectively.
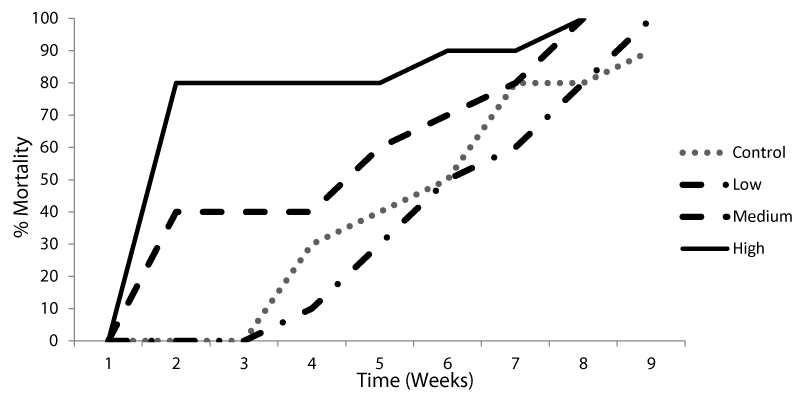
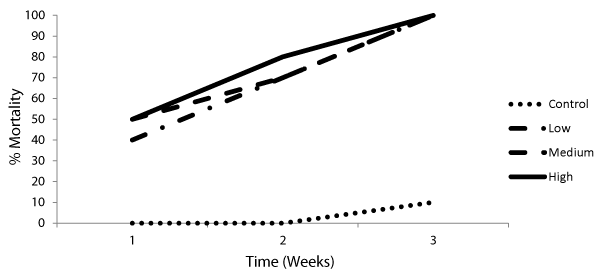
Mortality rates of the snails exposed to sub-lethal concentrations of BPA either for 24 hrs or for 7 days before miracidial exposure were shown in Figures 1,2. Results showed that exposure to different concentrations of BPA for 7 days before miracidial infection caused the death for the snails before reaching the patent period (cercarial output) as compared to the control group. Meanwhile, no cercarial output was recorded from snails exposed to BPA for 24 hrs till their death.
Discussion
Regarding the effect of environmental factors on the lethality values of BPA, results showed that temperature, water vegetation, bed mud and pH markedly affected the LC50 of BPA. A positive relationship was found between percentages of dead snails exposed to BPA and the temperature. This result was in agreement with those obtained by [17], who found that the molluscicidal activity of methanol extract of Adenium obesum plant against Bulinus truncatus snails was increased as the temperature increased.
Regarding the effect of different densities of L. Gibba and C. demersum on the mortality of B. alexandrina exposed to LC50 and LC90, results showed that the LC50 of BPA caused more mortality than applied LC50 value without plant. On the other hand, results showed that both plants have no effect on the LC90values at 1.0 and 2.0 g of both densities. This result could be explained as aquatic plants can rapidly absorb BPA through their roots from water and metabolize it into several glycosidic compounds. The glycosylation of BPA by plants leads to estrogenicity of the parent compound. Two oxidative enzymes, peroxidase and polyphenol oxidase, are closely associated with the BPA metabolism [27]. Reactive oxygen metabolites such as superoxide anion, hydroxyl radical, peroxyl radical, and hydrogen peroxide are cytotoxic agents because of their ability to induce oxidative stress [28]. BPA has been shown to induce oxidative stress in land and aquatic plants [29-31]. When the plants undergo decomposition; a large amount of nutrients (nitrogen, phosphorus) and organic material are released from the dying plants into the water causing death of snails.
Regarding the effect of different concentrations of bed mud on the values of LC50 and LC90of BPA against the snails, LC50 and LC90showed marked decrease in mortality of snails when mixed with 5.0 g L-1 of mud to reach to 30 % and 66.6 %, respectively. Increasing the concentration of mud to 10 mg L-1 increased the mortality of snails to be 70 % by using concentration of LC50 of PBA. This could be because the BPA had a moderate affinity for soil organic matter and is therefore unlikely to be mobile or bioavailable in soils than water column [32]. The half-life of BPA in soils has been estimated as 3 days [32], 7 days [33], and 37.5 days [34]. Increasing the concentration of mud caused increasing the toxicity of BPA on the snails. This could be explained as that much higher BPA concentrations in the sediments than in the upper water column [35]. It was noted a strong correlation between BPA levels near the base of the water column and those in the sediment [36].
The pH is an important factor in controlling the partitioning of BPA. Varying the pH of the water was achieved by adding 0.1 M HCl or 0.1 M NaOH. The effect of pH on mortility of snails was studied at different pH. Results showed that the toxicity of BPA against B. alexandrina differed by variation of the pH values of water. The percentage of dead snails was 50% at pH 7 however, the number of dead snails decreased under acidic and alkaline conditions. The obtained results were in agreement with those recorded a decrease in the molluscicidal activity of Agave filifera, Agave attenuate and Calendula micrantha in acidic and alkaline pH level against B. alexandrina snails [37].
It has been reported that the highest log Octanol-Water Partition Coefficient (Kow) occurred at approximately pH 8 [38]. The log Kow of BPA increased slightly at pH from 6 – 8 but decreased significantly at pH 10. A possible reason is the dissociation of BPA in the system, as pH 10 is within its pKa range (9.6 - 10.2) [39], in fact, BPA is a weak organic acid and can be deprotonated to exist in the system as an anionic form and/or neutral form of BPA. We can suggest that the acidic condition caused degradation to the BPA so, the toxicity of BPA decreased as the percentage of mortality of the snails decrease.
Regarding the effect of BPA toxicity on the survival rates of juvenile snails, results showed that the survival rate (Lx) of treated snails decreased during the experiments. The present findings can also be explained on the basis of storage depots as well as metabolic, physiological and biochemical hazardous effects of the accumulated doses. Our results are in accordance with those who found low concentrations of Atriplex halimus caused a sharp decline in the survival of B. alexandrina snail [40]. Moreover, it has been noticed that LC25 of both Hedera canariensis and Pittosorum tobira varigatum plants had more sharp effects than LC0 and LC10 on the survival rates of both juvenile and adult B. alexandrina snails [41].
It has been noticed that plant cells have the ability to synthesize many phenolic compounds and some of these are conjugated by glucosylation and accumulate in the vacuole [42]. This could be explained why the percentage of dead snails increase in the present of aquatic plants (L. Gibba) that more toxic than C. demersum.
Our findings reflected the important role of eggs age in determining the BPA toxicity against B. alexandrina. Hatchability rate showed a slight decrease upon exposure of 1-day-aged eggs to 0.097 and 0.485 mg L-1of BPA for one day while it decreased to 72.4% upon exposure to 0.97 mg L-1 BPA. In addition, the continuous exposure to BPA fora week caused a decrease of the hatchability rate of one- eggs and six-egg old where the 6-day-aged eggs was less affected when exposed to 0.97 mg L-1 of BPA either for one day or one week as compared to-day-aged eggs. This may be due to thicker gelatinous egg coat in 1-day-aged eggs than those of 6-day-aged .Several authors recorded a similar harmful and remarkable reduction in hatchability of B. alexandrina eggs treated with different chemicals [34,44].
A significant increase in the mortality rates of snails exposed to sublethal concentrations of the tested material compared to the control group was observed in the present study. This finding agrees with those showed marked reduction in the survival rate of snails treated with sublethal concentrations of different plant species compared to the control [45,46]. It was also noted that adult snails are more tolerant than juvenile one. Similarly, it has been noticed that some plant derived molluscicides caused a significant reduction in the fecundity and survival of young snails than adult snails [47,48].
Regarding the effect of BPA toxicity on egg laying capacity of adult snails, the exposure to lowest concentration (0.01 LC50) did not inhibit oogenesis or oviposition and recorded values showed an increase in the oviposition while increasing the concentration caused a complete disturbance in egg-laying capacity. Such reduction of snail’s fecundity may arise as a result of the action of the tested agent upon the steroid hormones, the harmful effect on the male and female genital tract, or may arise from metabolic disorders as has been described [49]. These findings are in a harmony with those results [50] showed that LC0, LC50 and LC10 of Oreopanax guatemalensis cause an elevation of egg production in B. alexandrina while LC25 cause complete retardation.
Regarding the possible effect of BPA on transmission of schistosomiasis, results showed that exposure to different concentration of BPA for 7 days before miracidial infection caused the death of all the snails before reaching the patent period (cercarial output) while, no cercarial output was recorded from snails exposed to BPA for 24 hrs till their death. This may be explained by the deteriorations of physiological parameters of snails making them unsuitable for the parasite development [46]. These results were in accordance with those found a reduction in infection rate with different Biomolluscicides [50-52].
In conclusion, our results showed that the environmental characteristics may alter the biological impacts of BPA and the exposure of snail to BPA may affect the transmission of schistosomiasis.
- Leal-Neto OB, Gomes ECS, Oliveira Junior FJM, Andrade R, Reis DL, et al. (2013) Biological and environmental factors associated with risk of schistosomiasis mansoni transmission in Porto de Galinhas, Pernambuco State, Brazil. Cad Saude Publica 29: 357-367.
- Keiser R, Adams B, Gasser D, Bazzi P, Dutre P, et al. (2005) A unified Lagrangian approach to solid fluid animation’, In Proceedings of Eurographics Symposium on Point-Based Graphics 125–133 .
- Morley NJ (2009) Environmental risk and toxicology of human and veterinary waste pharmaceutical exposure to wild aquaic host-parasite relationships’,Environ Toxicol Pharmacol 27: 161-175 .
- Chitra KC, Latchoumycandane C, Mathur PP (2002) Effect of nonylphenol on the antioxidant system in epididymal sperm of rats’, Arch Toxicol 76: 545-551 .
- Hernandez-Rodriguez G, Zumbado M, Luzard OP, Monterde JG, Blanco A, et al. (2007) ‘Multigenerational study of the hepatic effects exerted by the consumption of Haniokanonylphenol and 4-octylphenol contaminated drinking water in Sprague dawley rats’, Environ Toxicol Pharmacol 23: 73-81 .
- Oehlmann J, Schulte-Oehlmann U, Kloas W, Jagnytsch O, Lutz I, et al. (2009) A critical analysis of the biological impacts of plasticizers on wildlife.Philos Trans R Soc B 364: 2047-2062 .
- Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, et al. (2007) An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod Toxicol 24: 225-239 .
- US Environmental Protection Agency (USEPA, 2010) Bisphenol A action plan.Washington, DC. USA .
- Marcial HS, Hagiwara A, Snell TW (2003) Estrogenic compounds affect development of harpacticoid copepod Tigriopus japonicas. Environ Toxicol Chem 22: 3025-3030 .
- Watts, MM, Pascoe D, Carroll K (2003) Exposure to 17a- ethinyl estradiol and Bisphenol A-effects on larval moulting and mouthpart structure of Chironomus riparius.Ecotoxicol Environ Saf 54: 207-215 .
- Biggers WJ, Laufer H (2004) Identification of juvenile hormone-active alkylphenols in the lobster Homarus americanus and in marine sediments. Biol Bull 206: 13-24 .
- Oehlmann J, Schulte-Oehlmann U, Tillmann M, Markert B (2000) Effects of endocrine disruptors on prosobranch snails (Mollusca: Gastropoda) in the laboratory. Part 1: bisphenol A and octylphenol as xeno-estorgens.Ecotoxicol 9: 383-397 .
- Aarab N, Lemaire-Gony S, Unruh E, Hansen PD, Larsen BK, et al. (2006) Preliminary study of responses in mussel (Mytilus edulis) exposed to bisphenol A, diallyl phthalate and tetrabromodiphenyl ether’. Aquat Toxicol 78S: S86-S92 .
- El-Fiki SA Mohamed AM (1978) Effect of some herbicides on the toxicity of certain molluscicides against Biomphalaria alexandrina snails, Egypt. Egypt J Bilharz 5: 91-100 .
- Motoyama A, Suzuki A, Shirota O, Namba R (1999) Direct Determination of Bisphenol A and Nonylphenol in River Water by Column-switching Semi-microcolumn Liquid Chromatography/Electrospray Mass Spectrometry. Rapid Commun Mass Spectrom 13: 2204-2208.
- WHO (1965) Molluscicide screening and evaluation.Bull WHO 33: 567- 581 .
- Bakry FA, Mohamed, RT Hasheesh WS (2011) Impact of methanol extract of Adenium obesum plant on some biochemical and biological parameters of Bulinus truncates snails. J Evol Biol Res 3: 87-94 .
- El-Deeb FA (1996) Present status of environmental pollution by Niclosamide molluscicide used in Egypt’, Ph.D. Thesis, Agricultural Science Department, Institute of Environmental Studies and Research, Ain Shams Univ., Cairo.
- Dorn PB, Chou C, Gentempo JJ (1987) Degradation of bisphenol A in natural waters. Chemosphere 16: 1501-1507 .
- Frank GH (1963) Some factors affecting the fecundity of Biomphalaria pfeiffri (krauss) in glass aquaria, Bull WHO29: 531-537.
- Osman GY, Mohamed, AH, Sheir SK, Hassab EL-Nabi SE, et al. (2014) Molluscidal activity of Mirazid on Biomphalaria alexandrina snails: biological and molecular studies.Int J Adv Res 2: 977-989 .
- Oteifa B, Mousa A, Abou El – Hassan AA, Mohamed AM, et al. (1975) Effect of certain insecticides in the control of the fresh water snails; B. alexandrina and Bulinus trnucatus. J Bilharz 2: 221-243 .
- Osman GY, Mohamed AM, Abdel Kader A, Mohamed AA (2011) Biological studies on Biomphalaria alexandrina snails treated with Furcraea selloa marginata plant (family: Agavaceae) and Bacillus thuringiensis kurstaki (Dipel-2x). J App Pharm Sci 1: 47-55 .
- Abouel-Hassan AA, Zidan ZH, Massoud AA, El-Deeb FA (2000) Effect of sublethal concentrations of Niclosamide snailicide on the biotic and survival potential of Biomphalaria alexandrina snails.Arab Univ J Agric Sci8: 905-921 .
- Massoud J, Arafaa F, Chu KY (1973) Effect of Bayluscide (Bayer73) on the development of Schistosoma haematobium in Bulinus trancatus.Bull Soc Pathol Exot 66: 544-547 .
- Southwood TRE (1978) Ecological methods’, Halsted Press, Chapman and Hall. London. 524 .
- Kang JH, Katayama Y, Kondo F (2006) Biodegradation or metabolism of bisphenol A: from microorganisms to mammals. Toxicol 16: 81-90 .
- Dogan M, Korkunc M, Yunurtas O (2012) Effects of Bisphenol A and Tetrabromobisphenol A on Bread and Durum Wheat Varieties. Ekoloji 21: 114-122 .
- Li Y, Zhou Q, Li F, Liu X, Luo Y (2008) Effects of tetrabromobisphenol A as an emerging pollutant on wheat (Triticum aestivum) at biochemical levels. Chemosphere 74: 119-124 .
- Sun Y, Guo H, Yu H, Wang X, Wu J, et al. (2008) Bioaccumulation and physiological effects of tetrabromobisphenol A in coontail Ceratophyllum demersum L.’,Chemosphere 70: 1787-1795 .
- Liu Y, Guan Y, Gao Q, Tam NFY, Zhu W (2010) Cellular responses, biodegradation and bioaccumulation of endocrine disrupting chemicals in marine diatom Navicula incerta. Chemosphere 80: 592-599 .
- Fent G, Hein WJ, Moendel MJ, Kubiak R (2003) Fate of 14C-bisphenol A in soils. Chemosphere 51: 735-746 .
- Ying GG, Kookana RS (2005) Sorption and degradation of estrogen-like endocrine disrupting chemicals in soil. Environ Toxicol Chem 24: 2640-2645 .
- Environment Canada (2008) Screening Assessment for the Challenge: Phenol, 4,40-(1-Methylethylidene) bis-Bisphenol A. Chemical Abstracts Service Registry Number 80-05-7 .
- Flint S, Markle T, Thompson S Wallace E (2012) Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manage 15: 19-34 .
- Funakoshi G, Kasuya S (2009) Influence of an estuary dam on the dynamics of bisphenol A and alkylphenols. Chemosphere75: 491-497 .
- Abdel Kader A, Ramzy MT, Tantawy A (2004) Evaluation of the molluscicidal and in vitro schistosomicidal activity of butanol extract of the plant Agave filifera. Egypt J Biomed Sci 16: 53-67.
- Borrirukwisitsak S, Keenan HE, Gauchotte-Lindsay C (2012) Effects of Salinity, pH and Temperature on the Octanol-Water Partition Coefficient of Bisphenol A. IJESD 3: 460-464 .
- Staples CA, Dorn PB, Klecka GM, O’Block ST, Harris LR (1998) A Review of the Environmental Fate, Effects, and Exposures of Bisphenol A.Chemosphere 36: 2149-2173 .
- Tantawy AA (2002) Effect of sublethal concentrations of Atriplex halimus (Chnopediaceae) on Biomphalaria alexandrina, the snail- vector of Schistosoma mansoni in Egypt.J Egypt Soc Parasitol 32: 297-305 .
- El-Nahas HA, El-Deeb FA (2007) Molluscicidal potency of Pittosporum tobira vearigatum and Hedera canariensis plants against juvenile and adult Biomphalaria alexandrina snails. Egypt J Aquat Biol Fish 11: 151-170 .
- Kreuz K, Tommasini R, Martinoia E (1996) Old enzymes for a new job: herbicide detoxification in plants. Plant Physiol 111: 349–353 .
- Rizk ET, El-Mehlawy MH, Mona MH (2001) Evaluation of the slow release potency of polymeric niclosamide against B. alexandrina eggs and S. mansoni larvae. Egypt Ger Soc Zool 35: 189-204.
- Al-Mathal EM, Fouad MA (2006) Effect of C. molmol on adults, egg masses and egg-deposition of B. arabica under laboratory conditions. J Egypt Soc Parasitol 36: 305-314 .
- Ahmed T Sharaf El-Din, Favez A Bakrv, Ahmed A Tantawy(2001) Molluscicidal activity of Zygophyllum simplex (Family; Zygophyllaceae) against Biomphalaria alexandrina and Bulinus truncates. Egypt J Aquat Biol Fish 4: 131-143 .
- Gawish F, Mossalem H, El-Einin AH (2008) Bioassay of the plant Callistemon citriun against Bulinus trancatus snails and their infection with Schistosoma haematobium. Egypt J Schistosomiasis Infect Endem Dis 30: 33-41.
- Anto F, Aryeetey ME, Anyorigiya T, Asoala V, Kpikpi J (2005) The relative susceptibilities of juvenile and adult Bulinus globosus and Bulinus truncatus to the molluscicidal activities in the fruit of Ghanaian Blighia sapida, Blighia unijugata and Balanites aegyptiaca’. Ann Trop Med Parasitol 99: 211-217 .
- Singh P, Singh VK, Singh DK (2005) ‘Effect of binary combination of some-plant derived molluscicides with MGK-264 or piperonylbutoxide on the reproduction of the snail Lymnaea acuminate. Pest Manag Sci 67: 204-208 .
- Mohamed AM, El-Fiki SA, El-Sawy MF, El-Wakil H (1981) Effect of prolonged exposure of B. alexandrina to low concentrations of some molluscicides.J Egypt Soc Parasitol 11: 295-311 .
- Hasheesh WS, Marie MAS, El-Deeb FAA, Sayed SSM (2011) Impact of Asparagus densiflours and Oreopanax Guatemalensis plants and Difenoconazole Fungicide on Biochemical Parameters of Biomphalaria alexandrina Snails. Aust J Basic ApplSci 5: 366-378 .
- Bakry FA Abd-El-Monem S (2005) Effect of water plants and non-target snails on the infectivity of Bulinus truncatus with Schistosoma haematobium’. J Egypt Soc Parasitol 35: 859-874.
- Bakry FA, Abdel-Hamid H, Abu El Einin HM (2007) Effect of neem plant (Azadirachta indica) on some biological and histological parameters of non-infected Biomphalaria alexandrina and infected with Schistosoma mansoni. J Egypt Ger Soc Zool54D: 51-68.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
