International Journal of Veterinary Science and Research
Comparative Anthelmintic Efficacy of Ivermectin and Levamisole in Dogs Naturally Infected with Gastrointestinal Nematodes in Bishoftu, Central Ethiopia
1Ethiopian Biodiversity Institute (EBI), P.O. Box: 30726, Addis Ababa, Ethiopia
2Mekdela District Livestock Resource Development Office, Masha, Ethiopia
Author and article information
Cite this as
Klepikov I. Comparative Anthelmintic Efficacy of Ivermectin and Levamisole in Dogs Naturally Infected with Gastrointestinal Nematodes in Bishoftu, Central Ethiopia. Int J Vet Sci Res. 2025; 11(3): 027-033. Available from: 10.17352/ijvsr.000155
Copyright License
© 2025 Klepikov I. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Background: Gastrointestinal helminths are among the most common causes of diseases in dogs, livestock, and zoonotic infections. Despite the widespread distribution of parasitic diseases in dogs, they have received very little attention in Ethiopia. Moreover, the improper use of drugs has resulted in ineffective control of helminths and anthelmintic resistance. Thus, an experimental study was conducted to determine the comparative anthelmintic efficacy of Ivermectin and Levamisole in dogs naturally infected with gastrointestinal nematodes.
Methods: The experiment was conducted on 180 dogs identified with at least 100 eggs per gram of feces and randomly assigned into three treatment groups. Treatment Group I was treated with Ivermectin, Treatment Group II was treated with Levamisole, and Treatment Group III was the control. Fecal samples were collected on day 0 and day 14. Fecal egg count reduction analysis was conducted to determine the efficacy of anthelmintic drugs.
Results: Among 180 dogs, the mean fecal egg count was 1249.02 (95% CI: 1139.57–1358.47) at day 0. This study indicated a significant difference (p < 0.05) in the mean fecal egg count among the treatment groups at day 14. The mean fecal egg count was reduced to 37.9 (95% CI: 20.93–54.87) and 88 (95% CI: 57.54–118.46) in dogs treated with Ivermectin and Levamisole, respectively. The fecal egg count reduction analysis indicated significant reductions in fecal egg count in dogs treated with anthelmintics at day 14. The fecal egg count reduction was 97.15% (95% CI: 91.12–99) and 93.23% (95% CI: 84.29–98) in dogs treated with Ivermectin and Levamisole, respectively.
Conclusions: This study demonstrates the satisfactory efficacy of Ivermectin, whereas Levamisole was suspected of resistance in the treatment of gastrointestinal nematodes in dogs at the recommended dosage. Thus, the present study suggests Ivermectin treatment, a combination of Levamisole, and further studies on anthelmintics for dogs against gastrointestinal helminths.
ANOVA: Analysis of Variance; EPG: Egg Per Gram; FEC: Fecal Egg Count; FECR: Fecal Egg Count Reduction; GIT: Gastrointestinal Tract
Introduction
Pets are companion animals that play indispensable roles in human life, providing several benefits to mental health and social well-being. Dogs are closely associated with humans and are kept for companionship, house guard, hunting, and security purposes [1]. Despite their countless benefits, dogs harbor parasitic infections, which can cause serious health problems in dog and zoonotic risks, including helminths and protozoa, that contaminate the environme and transmit to livestock and humans [2]. Dogs are infected with several parasitic diseases, particularly gastrointestinal (GIT) nematodes. Canine gastrointestinal parasites are a global health problem, particularly in developing countries, and are of great concern to environmental and public health [3].
Gastrointestinal parasites are the most common cause of infectious gastrointestinal disease in dogs. Endoparasites cause different clinical signs in dogs, including anorexia, anemia, unthriftiness, poor growth, and several GIT disorders, such as diarrhea [4,5]. The most common canine GIT helminths include Ascarids and Ancylostomatidae, which are associated with considerable morbidity and mortality rates, especially in the young. Toxocara canis, Ancylostoma caninum, Trichuris vulpis, and Dipylidium caninum are the main GIT parasites of dogs with global significa. Most importantly, canine endoparasites, such as Toxocara canis, Ancylostoma caninum, and Dipylidium caninum, pose a potential zoonotic threat. Pets usually excrete helminth eggs or larvae in their feces into the environment and transmit the diseases to animals and humans [6].
Anthelmintics are commonly used to treat parasitic infections in dogs. There are different anthelmintics used to treat endoparasites, either by stunning or killing them without causing significant damage to the host [7]. The most important drugs used to treat parasitic infestations in pets include ivermectin, praziquantel, piperazine, and levamisole. Most of the drugs are broad-spectrum anthelmintics acting on different endoparasitic species [8]. Ivermectin is one of the most commonly used broad-spectrum antiparasitic agents against nematodes and exoparasites. It is a semisynthetic macrocyclic lactone effective against most common internal parasites, except tapeworms and trematodes [9]. On the other hand, levamisole is an anthelmintic agent used to treat and control a wide range of nematodes in livestock species and as a microfilaricide in pets. Most anthelmintics and nematicides are limited in their action against trematodes, cestodes, and nematodes [10].
Despite the availability of various anthelmintic medications to treat and control parasites, the ubiquitous and prolific nature of helminths makes their elimination challenging. Moreover, anthelmintic resistance has been developed due to the improper use of drugs or parasitic genetic modifica. In Ethiopia, dogs are important animals in many urban and rural households, mostly as companion pets and houseguards. However, very little attention has been given to the parasitic diseases of dogs and the control of canine helminths in the country. Besides, the improper use of drugs has resulted in ineffective control of helminths in pets and parasitic anthelmintic resista. This study selected ivermectin and levamisole for comparative anthelmintic efficacy evaluation, as the drugs are the commonly used anthelmintics in the treatment of parasitic infections in pets in Ethiopia, possess different mechanisms of action, and have various spectra of action. Therefore, this study was designed to determine the comparative efficacy of the most commonly used anthelmintics, ivermectin and levamisole, in dogs naturally infected with gastrointestinal nematodes in Bishoftu, central Ethiopia.
Materials and methods
Description of the study area
The study was conducted on the dog population in Bishoftu town in central Ethiopia. Bishoftu is a city found in the Eastern Shewa zone of the Oromia Regional State. The town is located 45 km southeast of Addis Ababa at 8°35’ N latitude and 39°06’ E longitude, with an altitude of 1850 m above sea level (Figure 1). Bishoftu is well-known for its multiple Great Rift Valley lakes [11]. The climatic condition is characterized by bimodal rainfall, with average annual rainfall and a temperature of 892 mm and 20 ºC, respectively. Dogs are kept by households in the urban, peri-urban, and rural areas of Bishoftu. Many dog helminths are endemic in the area and pose inevitable threats to human and animal health [12].
Study animals
The dog population kept as pets in Bishoftu town, particularly the dogs presented to a veterinary teaching hospital of the College of Veterinary Medicine and Agriculture of Addis Ababa University, was used as study animals. The study animals include dogs of various age categories, dog breeds, and both sexes (male and female). The age was categorized as young in dogs below 1 year old and adults in dogs above 1 year old [11]. In Bishoftu town and the surrounding areas, there are different breeds of dogs, including local dogs, crossbred dogs, and exotic dog breeds. The body condition was characterized as poor, medium, and good based on the physical appearance and manual palpation of the ribs and transverse processes. Dogs were kept in different management systems, where some were entirely confined and the others were semi-confined [13].
Study design, sampling methodology, and sample size determination
The study was based on fecal parasitic egg determination pre- and post-treatment with anthelmintic drugs. A purposive sampling strategy was employed to select the constituents of the study animals. The dogs brought to the veterinary teaching hospital of the College of Veterinary Medicine and Agriculture, Addis Ababa University, and veterinary clinics for rabies mass vaccination were randomly selected for parasitic diagnosis and fecal egg count. The study comprised dogs that tested positive for gastrointestinal nematodes and had at least 100 eggs per gram of feces [14]. The study included a total of 180 dogs positive for gastrointestinal nematodes on a fecal egg test [15]. The selected dogs were classified based on sex (male and female), age (young, adult, and old), and breed (local and exotic), and finally randomly allocated to the three treatment groups from each stratum.
Sample collection and laboratory examination
Fecal samples were collected using the universal plastic bottle directly from the rectum or immediately after defecation. About 6–8 grams of the fecal sample were collected from each sampled animal. The fecal samples were labeled and preserved with 10% formalin and transported to the laboratory for fecal egg examination [12]. The fecal laboratory examination was conducted at the parasitological laboratory of the College of Veterinary Medicine and Agriculture of Addis Ababa University (CVMA-AAU). In this study, fecal sample collection and microscopic examination of parasitic eggs were performed on days 0 (pre-treatment) and 14 (post-treatment). Animal-level risk factors, including age, body condition, and sex, were recorded along with fecal sample collection.
The laboratory examination was conducted according to the parasitological protocols for fecal egg counts (FEC). Three grams of fecal samples were weighed on a sensitive balance and thoroughly mixed with 42 ml of flotation fluid in the mortar. The feces were crushed with a pestle in the flotation fluid for a homogenized solution. The solution was filtered with a sieve to remove debris. The filtrate was centrifuged at 3000 rpm for 3 minutes. The top-up of the flotation fluid was added to the centrifuge tube until a cone shape was formed at the top. Then, finally, a coverslip was placed on the top of the tube and allowed to stand for 15-20 minutes [12,16]. The coverslip was gently raised and placed on the microscope slide for microscopic examination and FEC. The total number of eggs counted times 100 represented the number of eggs per gram (EPG) of feces. Since approximately 0.15 ml of the suspension was uwhich is 1/300 of the 45 ml fecal-flotation fluid suspension (42 ml water and 3 grams of feces), then the number of eggs in 0.15 ml times 100 is equal to 1/3 of the total number of eggs in the measured 3 grams of feces. The slides were examined under 10X resolution of the objective lens for helminth eggs, and counted by the Stoll egg counting technique under a light microscope [17].
Experimental design
The study was designed by randomly allocating dogs positive for gastrointestinal nematodes into three experimental treatment groups: Treatment Group I, Treatment Group II, and Treatment Group III. Accordingly, 75 positive dogs were included in Treatment Group I and Treatment Group II, while 30 dogs were considered in Treatment Group III. Dogs in Treatment Group I received ivermectin anthelmintic treatment, while dogs in Treatment Group II were treated with levamisole. Treatment Group III was a control group, and the dogs in the group had not received any anthelmintic treatment. Ivermectin is a class of macrocyclic lactones and a broad-spectrum anthelmintic. It is a widely used antiparasitic medication for dogs in the treatment of a broad range of parasites, particularly effective against nematodes and ectoparasites. Levamisole is a broad-spectrum anthelmintic effective against roundworms and is commonly used in treating and controlling endoparasites in dogs [18]. Ivermectin was administered at a dosage of 0.2 mg/kg in the subcutaneous route, while levamisole was orally administered at a 5 mg/kg dosage (Table 1).
Faecal egg count reduction analysis
The ability of parasites to survive treatments that are generally effective at the recommended doses is a major threat to the future control of worm parasites in dogs. The efficacy is measured by the “fecal egg count reduction test” value, which varies for different types of helminth treatment. The fecal egg count reduction test (FECRT) was conducted to evaluate the anthelmintic efficacy and resistance. It is commonly used as a screening test for veterinarians and producers to identify the desired clearance level of the parasites after anthelmintic treatment. The efficacy of the drugs was tested according to the World Association for the Advancement of Veterinary Parasitology (WAAVP) recommendations for the detection of anthelmintic resistance in animals by the percentage reduction of mean egg excafter the post-treatment. FECR% = 100 (1 − 𝑋𝑡/𝑋𝑐), where 𝑋𝑡 and 𝑋𝑐 are the arithmetic means of EPG in the treated (t) and control (c) groups at day 14 post-treatment. Resistance or reduced efficacy (R) is present if FECR < 90% and the lower limit of the 95% confidence interval < 90%; resistance is suspected (S) or doubtful efficacy if FECR ≥ 90% and the lower limit of the 95% confidence interval < 90%; and no resistance (N) or satisfactory efficacy if FECR ≥ 90% and the lower limit of the 95% confidence interval > 90% [20].
Data management and analysis
The data collected from the field and laboratory exawere entered into a Microsoft Excel spreadsheet. The many attribute data include animal-level risk factors, fecal egg count on pre- and post-treatment, and fecal egg count reduction results. The data was analyzed with R statistical software version 4.1.2. They were summarized using descriptive statistics (means, standard deviation, and reduction percentages). The effectiveness of the different anthelmintics was evaluated by computing the mean fecal egg count reduction for each treatment group. The reduction in FEC post-treatment was calculated using 100 (1- Xt/Xc), where the Xt arithmetic mean of the post-treatment egg count on the 14th day, and the Xc arithmetic mean of the control group on the 14th day [19]. The log transformation of the values of EPG [using log (x + 1)] was performed to minimize and stabilize the variance. The one-way ANOVA analysis was used to compare the mean EPG among the experimental groups.
Results
Pre-treatment fecal egg count
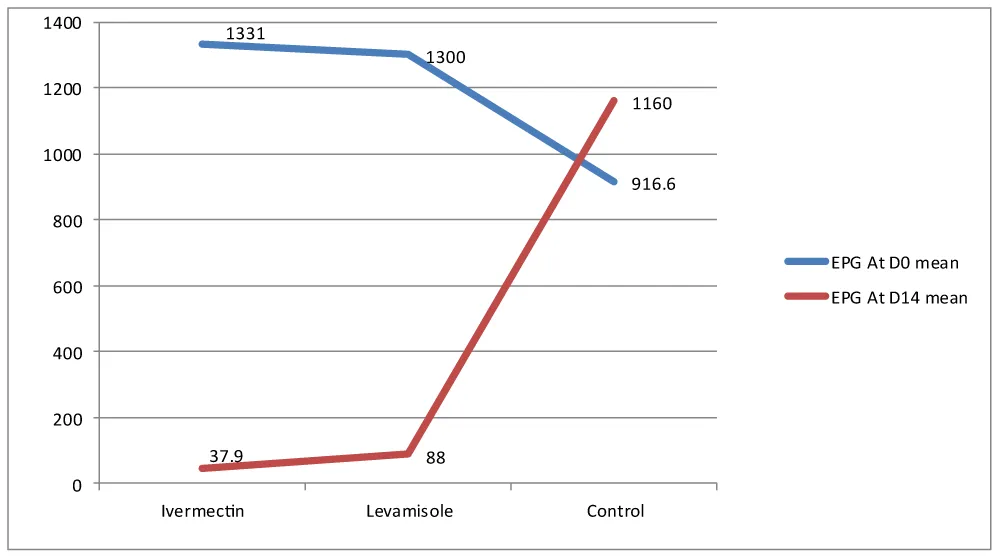
This anthelmintic efficacy study involved 180 dogs naturally infected with gastrointestinal nematodes, divided into three experimental groups. The mean fecal egg count (FEC) at day 0 (pre-treatment) was 1249.02 (95% CI: 1139.57–1358.47). The study determined the highest pre-treatment fecal egg count in Treatment Group I with a mean EPG of 1331 (95% CI: 1165.56–1496.44). At day 0, the mean FEC was 1300 (95% CI: 1142.32–1457.68) EPG in Treatment Group II. Accordingly, the mean pre-treatment fecal egg count in Treatment Group III was 916.6 (95% CI: 623.17–1210.03) EPG (Table 2). The results indicated that the mean EPG in Treatment Group III was the lowest fecal egg count among the treatment groups.
ANOVA analysis of post-treatment fecal egg count
The mean fecal egg count at day 14 indicated the significance of anthelmintic drugs against gastrointestinal nematodes. Among 75 dogs treated with the Ivermectin anthelmintic drug, 71% were negative for GIT nematodes at day 14. Accordingly, 68% of dogs that received Levamisole anthelmintic treatmeof fecal egg count for GIT nematodes. However, all dogs not treated with anthelmintics were positive for GIT nematodes (mean EPG: 1160; 95% CI: 801.95 – 1,518.1). In this study, 58% (104/180) of the dogs were diagnosed negative for every GIT nematode under investigation (Table 3).
The one-way ANOVA analysis revealed a significant difference (p < 0.05) in the mean fecal egg counts among treatment groups. Dogs treated with Ivermectin had a mean fecal egg count of 37.9 EPG (95% CI: 20.93-54.87). While those treated with Levamisole had a mean of 88 EPG (95% CI: 57.54-118.46). The control group had a significantly higher mean fecal egg count of 1160(95% CI: 801.95-1518.1) compared to both anthelmintic treatment groups. By day 14, the total mean fecal egg count decreased to 246.46 EPg (95% CI: 187.53-305.39) (Table 3).
Fecal egg count reduction analysis
The fecal egg count reduction (FECR) analysis indicated a significant decrease in mean fecal egg count in dogs treated with anthelmintics compared to the control groups. Dogs treated with Ivermectin experienced a 97.15% reduction in egg per gram (EPG) at day 14 (95% CI: 91.12–99). Levamisole treatment led to a 93.23% reduction (95% CI: 84.29–98). In contrast, the control group saw an increase in mean fecal egg count from 916.6 EPG on day 0 to 1160 EPG on day 14, with no FECR recorded (Table 4).
The comparative efficacy study indicated that Ivermectin was effective as an anthelmintic treatment, while Levamisole raised concerns about potential drug resistance. The FECR was higher for dogs treated with Ivermectin (97.15%) compared to those receiving Levamisole (93.23%) (Table 4). Ivermectin treatment decreased the mean fecal egg count from 1331 EPG on day 0 (95% CI: 1165.56–1496.44) to 37.9 EPG on day 14 (95% CI: 20.93–54.87). Although Levamisole showed signs of resistance, it still reduced the mean fecal count from 1300 EPG pre-treatment to 88 EPG post-treatment. Both anthelmintics significantly lowered fecal egg counts compared to the control group (Figure 2).
Discussion
The present study assessed the efficacy of anthelmintic drugs on dogs naturally infected with gastrointestinal nematodes, revealing a mean fecal egg count (FEC) of 1249.02 EPG at day 0. Similar findings of higher FEC in dogs were reported in Rwanda [2], Brazil [21], and New Jersey [22]. Additionally, Dubie, et al. [23] and Tadesse, et al. [12] noted a higher prevalence of GIT helminths (Strongyloides species, Ancylostoma caninum, Trichuris vulpis, and Toxocara canis) in different parts of Ethiopia. The high prevalence and parasitic fecal egg count suggest inadequate pet management practices in Bishoftu town, such as irregular deworming, feeding of raw animal products, and environmental contamination.
This research revealed a significant difference (p < 0.05) in mean fecal egg count at day 14 among the treatment groups. The mean FEC was highly reduced in treatment groups that received anthelmintic treatments as compared to the control group. Similarly, several studies [24,25] reported the significant reduction of fecal egg count in animals treated with anthelmintics. In this study, about 71% of the dogs treated with Ivermectin anthelmintics and 68% of the dogs receiving Levamisole treatment were diagnosed free of any GIT nematodes. Accordingly, significant helminth clearance was reported in dogs treated with Levamisole [10], in dogs receiving Ivermectin anthelmintics against the GIT nematodes [26], and in working equids treated with Ivermectin [27]. In contrast, Solomon, et al. [28] reported low parasitic clearance in sheep treated with Ivermectin. The variation in the findings could be due to the difference in GIT nematode species, drug dosage, and parasitic resistance. The present study indicated the inevitable role of anthelmintics, particularly Ivermectin and Levamisole, in protecting animals from GIT nematodes.
The current study revealed that the fecal egg count reduction (FECR) was 97.15% (95% CI: 91.12–99) in dogs treated with Ivermectin at day 14. In line with this finding, the FECR of 98.02% in dogs with GIT nematodes [26], 99.7% in horses [29], 97.7% in sheep [19], and 95% in equine strongylids [30] were reported in different countries. However, some studies reported a lower FECR for Ivermectin treatment in various species infected with gastrointestinal helminths [24]. The variations in the findings might be seen from the differences in types of gastrointestinal, parasites and experimental animal species. The current finding revealed that Ivermectin anthelmintic treatment was protective in dogs against GIT nematodes if provided at the recommended dosage.
In the present finding, the mean fecal egg count was reduced by 93.23% (95% CI: 84.29–98) in dogs that received Levamisole treatment. Similar findings of 92% in helminths of dogs [10], 90% in cattle nematodes in Brazil [31], and 96% in African dwarf goats [32] were reported. In contrast, Carolina, et al. [33] reported a lower FECR (70.4 %) in a sheep flock treated with Levamisole. The difference in this study might be due to the drug dosage, route of administration, and the GIT nematode species encountered. The FECR in the current study was above 90% and indicated the possible treatment option of canine gastrointestinal nematodes. Moreover, the drug could be used in combination with other anthelmintics, for specific helminth species, or in different formulation types, dosages, and routes of drug administration.found that Ivermectin anthelmintic treatment has satisfactory efficacy against gastrointestinal nematodes in dogs at the recommended dosage. In line with this finding, Ivermectin treatment was indicated as effective in gastrointestinal nematode-infected dogs [26], in equines [27], in sheep [35], and in the GIT nematodes of sheep [36]. However, in this study, Levamisole was suspected of causing parasitic resistance to treating gastrointestinal nematodes in dogs. Accordingly, several studies [33] indicated the lowered efficacy of Levamisole in the treatment of parasitic diseases. In contrast, studies conducted on nematode infections of dogs [37], in goats [32], and against GIT nematodes of sheep [28] indicated significant efficacy of Levamisole. The present finding showed the significant efficacy of Ivermectin over Levamisole at the recommended dosage in treating gastrointestinal nematodes in dogs [38-42].
Conclusion
The present study demonstrated a satisfactory efficacy of Ivermectin anthelmintic treatment against gastrointestinal nematodes in dogs. Ivermectin treatment via the subcutaneous route significantly reduced the fecal egg count on day 14 at the recommended dosage rate. Dogs treated with Levamisole anthelmintic through the oral route indicated a significant reduction in the mean fecal egg count. However, this study revealed a suspected resistance to Levamisole treatment against gastrointestinal nematodes in dogs at the recommended dosage rate. The current results showed the inevitable effects of anthelmintics in the treatment of gastrointestinal nematodes in dogs. However, the study detected anthelmintics possessing different efficacy levels against gastrointestinal nematodes. Thus, this study suggests the proper use of Ivermectin treatment, a combination of levamisole treatment with anthelmintics, and further studies on anthelmintics and dosage rates for the treatment and control of gastrointestinal nematodes, in dto protect animal and public health.
Ethical approval and informed consent
The animal research ethical review committee of the College of Veterinary Medicine and Agriculture of Addis Ababa University granted ethical approval for this study. The dogs were handled following the best veterinary care guidelines during sample collection and treatments. Before conducting the research, the dog owners were informed about the objectives and benefits of the study. Written consent was obtained from animal owners and approved by the ethical committee of the College of Veterinary Medicine and Agriculture of Addis Ababa University. The study includes dogs whose owners gave consent for their animals’ inclusion in the study.
Availability of data and materials
The data collected and used to support this article can be offered by the corresponding author upon request.
Funding
This study was financially supported by the College of Veterinary Medicine of Addis Ababa University of Ethiopia. The funder had no role in the conception, design of the study, data collection, analysis, and interpretation of the data reported in this manuscript.
Authors’ contributions
Aweke Engdawork: Writing - original draft, Methodology, Investigation, Formal Analysis, Data curation, Conceptualization, Writing – review and editing, and Approving the final version.
Fikir Tizazu: Writing-original draft, Methodology, Investigation, Formal Analysis, Data curation, and Conceptualization.
The authors would like to acknowledge the College of Veterinary Medicine and Agriculture of Addis Ababa University for its technical and financial support during the research work. We acknowledge the dog owners for their willingness and consent to participate in the study. We are also grateful to all who provided us with invaluable support, guidance, and critical manuscript appraisal.
- Brooks HL, Rushton K, Lovell K, Bee P, Walker L, Grant L, et al. The power of support from companion animals for people living with mental health problems: a systematic review and narrative synthesis of the evidence. BMC Psychiatry. 2018;18(1):1–12. Available from: https://doi.org/10.1186/s12888-018-1613-2
- Ntampaka P, Niragire F, Nyaga PN, Habarugira G. Canine gastrointestinal nematodiases and associated risk factors in Kigali City, Rwanda. J Parasitol Res. 2021;2021:1–8. Available from: https://doi.org/10.1155/2021/9956256
- Ilić T, Nišavić U, Gajić B, Nenadović K, Ristić M, Stanojević D, et al. Prevalence of intestinal parasites in dogs from public shelters in Serbia. Comp Immunol Microbiol Infect Dis. 2021;76:1–8. Available from: https://doi.org/10.1016/j.cimid.2021.101653
- Christina L, Arwid M, Cora D. Gastrointestinal parasites in young dogs and risk factors associated with infection. Parasitol Res. 2023;122:585–96. Available from: https://doi.org/10.1007/s00436-022-07760-9
- Kohansal MH, Fazaeli A, Nourian A, Haniloo A, Kamali K. Dogs’ gastrointestinal parasites and their association with public health in Iran. J Vet Res. 2017;61:189–95. Available from: https://doi.org/10.1515/jvetres-2017-0024
- Pal M, Garedaghi Y, Tolawak D. A comprehensive review on major zoonotic parasites from dogs and cats. Int J Med Parasitol Epidemiol Sci. 2023;4(1):3–11. Available from: https://doi.org/10.34172/ijmpes.2023.02
- Hostetler J, Jimenez PD, Mansour A, Charles S, Hostetler J. Efficacy evaluation of anthelmintic products against an infection with the canine hookworm (Ancylostoma caninum) isolate Worthy 4.1F3P in dogs. Int J Parasitol Drugs Drug Resist. 2020;13:22–7. Available from: https://doi.org/10.1016/j.ijpddr.2020.04.003
- Nixon SA, Welz C, Woods DJ, Costa-junior L, Zamanian M, Martin RJ. Where are all the anthelmintics? Challenges and opportunities on the path to new anthelmintics. Int J Parasitol Drugs Drug Resist. 2020;14:8–16. Available from: https://doi.org/10.1016/j.ijpddr.2020.07.001
- Rodríguez-Morales AJ, Díaz-Vélez C, Apolaya-Segura M, Valladares-Garrido MJ. Evidence-based indications for ivermectin in parasitic diseases: an integrated approach to context and challenges in Peru. Parasite Epidemiol Control. 2023;23:1–7. Available from: https://doi.org/10.1016/j.parepi.2023.e00320
- Gabriel A, Ofemile YP, Philip AM. Levamisole efficacy on the Helminths in the National Veterinary Research Institute, Vom (NVRI) environment. Am J Biochem Mol Biol. 2020;10:45–50. Available from: https://doi.org/10.3923/ajbmb.2020.45.50
- Gutema FD, Yohannes GW, Abdi RD, Abuna F, Ayana D, Waktole H, et al. Dipylidium caninum infection in dogs and humans in Bishoftu. Diseases. 2021;9(1):1–7. Available from: https://www.mdpi.com/2079-9721/9/1/1
- Tadesse M, Ayana D, Kumsa B, Fromsa A. Zoonotic helminth parasites of dogs in Bishoftu Town, central Ethiopia: prevalence, dog owners’ knowledge and control practice. Ethiop Vet J. 2020;24(1):93–115. Available from: https://dx.doi.org/10.4314/evj.v24i1.7
- Warembourg C, Wera E, Odoch T, Bulu PM, Berger-González M, Alvarez D, et al. Comparative study of free-roaming domestic dog management and roaming behavior across four countries: Chad, Guatemala, Indonesia and Uganda. Front Vet Sci. 2021;8:617900. Available from: https://doi.org/10.3389/fvets.2021.617900
- Stafford K, Kollasch TM, Duncan KT, Horr S, Goddu T, Loomer CH, et al. Detection of gastrointestinal parasitism at recreational canine sites in the USA: the DOGPARCS study. Parasites Vectors. 2020;13(275):1–10. Available from: https://doi.org/10.1186/s13071-020-04147-6
- Traversa D, Joachim A. The 3Rs concept: time to change how we evaluate the efficacy of anthelmintics in companion animals. Trends Parasitol. 2018;34(1):41–52. Available from: https://doi.org/10.1016/j.pt.2017.09.002
- Sabatini GA, Borges FDA, Claerebout E, Gianechini LS, Höglund J, Kaplan RM, et al. Practical guide to the diagnostics of ruminant gastrointestinal nematode, liver fluke and lungworm infection: interpretation and usability of results. Parasites Vectors. 2023;16(58):1–17. Available from: https://doi.org/10.1186/s13071-023-05680-w
- Ngwese MM, Manouana P, Alvyn P, Moure N. Diagnostic techniques of soil-transmitted helminths: impact on control measures. Trop Med Infect Dis. 2020;5(2):93. Available from: https://doi.org/10.3390/tropicalmed5020093
- Getahun M, Tefera B, Bacha B, Eticha T. Quality of veterinary anthelmintic drugs marketed in Gondar Zone, North West Ethiopia: presence of poor-quality medicines. Heliyon. 2023;9(7):e18023. Available from: https://doi.org/10.1016/j.heliyon.2023.e18023
- Seyoum Z, Demessie Y, Bogale B, Melaku A. Field evaluation of the efficacy of common anthelmintics used in the control of gastrointestinal nematodes of sheep in Dabat district, Northwest Ethiopia. BMC Vet Res. 2017;13:1–8. Available from: https://doi.org/10.1186/s13620-017-0097-6
- Kaplan RM, Denwood MJ, Nielsen MK, Thamsborg SM, Torgerson R, Gilleard JS, et al. WAAVP guideline for diagnosing anthelmintic resistance using the faecal egg count reduction test in ruminants, horses, and swine. Vet Parasitol. 2023;318:109936. Available from: https://doi.org/10.1016/j.vetpar.2023.109936
- Jesus AP de, Holsback L, Fertonani S. Efficacy of pyrantel pamoate and ivermectin for the treatment of canine nematodes. Semin Cienc Agrar. 2015;36(6):3731–40. Available from: https://doi.org/10.5433/1679-0359.2015v36n6p3731
- Balk JD, Mitchell ND, Hughes J, Soto P, Rossi J, Ramirez-Barrios R. Multiple anthelmintic drug-resistant Ancylostoma caninum in foxhounds. Int J Parasitol Drugs Drug Resist. 2023;22:102–6. Available from: https://doi.org/10.1016/j.ijpddr.2023.07.001
- Dubie T, Sire S, Fentahun G, Bizuayehu F. Prevalence of gastrointestinal helminths of dogs and associated factors in Hawassa City of Sidama Region, Ethiopia. J Parasitol Res. 2023;2023:1–7. Available from: https://doi.org/10.1155/2023/6155741
- Gasbarre LC, Ballweber LR, Stromberg BE, Dargatz DA, Rodriguez JM, Kopral CA, et al. Effectiveness of current anthelmintic treatment programs on reducing fecal egg counts in United States cow-calf operations Résumé. Can J Vet Res. 2015;79:296–302. Available from: https://pubmed.ncbi.nlm.nih.gov/26424910/
- Jimenez PD, Mansour A, Charles S, Hostetler J, Settje T, Kulke D, et al. Efficacy evaluation of anthelmintic products against an infection with the canine hookworm (Ancylostoma caninum) isolate Worthy 4.1F3P in dogs. IJP Drugs Drug Resist. 2020 Apr;13:22–7. Available from: https://doi.org/10.1016/j.ijpddr.2020.04.003
- Panigrahi PN, Gupta AR, Patra RC, Mohanty BN, Maiti A, Sahoo GR. Comparative anthelmintic efficacy of ivermectin delivered through different routes in gastrointestinal nematode-infected dogs. Vet World. 2014;7(4):295–8. Available from: https://doi.org/10.14202/VETWORLD.2014.295-298
- Fesseha H, Mathewos M, Kidanemariam F. Anthelmintic efficacy of Strongyle nematodes to ivermectin and fenbendazole on working donkeys (Equus asinus) in and around Hosaena To, Southern Ethiopia. 2020. Available from: https://doi.org/
- Solomon L, Haile G, Ahmed NA, Abdeta D, Galalcha W, Hailu Y. Epidemiology and field efficacy of anthelmintic drugs associated with gastrointestinal nematodes of sheep in Nejo district, Oromia, Ethiopia. Sci Rep. 2024;14:1–10. Available from: https://doi.org/10.1038/s41598-024-55611-7
- Nielsen MK, Finnerty CA, Ripley NE, Page AE, McClendon ME, Adams AA. Ivermectin performance in horses diagnosed with equine endocrine disorders. Vet Parasitol. 2024;328(4):182. Available from: https://doi.org/10.1016/j.vetpar.2024.110182
- Nielsen KM, Littman BA, Orzech SW, Ripley NE. Equine strongylids: Ivermectin efficacy and fecal egg shedding patterns. Parasitol Res. 2022;121:1691–7. Available from: https://doi.org/10.1007/s00436-022-07509-4
- Soutello RGV, Seno MCZ, Amarante AFT. Anthelmintic resistance in cattle nematodes in northwest São Paulo State, Brazil. Vet Parasitol. 2007;148:360–4. Available from: https://doi.org/10.1016/j.vetpar.2007.06.023
- Adediran OA, Uwalaka EC. Effectiveness evaluation of levamisole, albendazole, ivermectin, and Vernonia amygdalina in West African Dwarf goats. J Parasitol Res. 2015;2015:1–5. Available from: https://doi.org/10.1155/2015/706824
- Carolina A, Chagas DS, Domingues LF, Gaínza YA, Barioni-Júnior W, Esteves SN. Target selected treatment with levamisole to control the development of anthelmintic resistance in a sheep flock. Parasitol Res. 2016;115:1131–9. Available from: https://doi.org/10.1007/s00436-015-4844-x
- Fissiha W, Kinde MZ. Anthelmintic resistance and its mechanism: A review. Infect Drug Resist. 2021;14:5403–10. Available from: https://doi.org/10.2147/idr.s332378
- Puspitasari S, Farajallah A, Sulistiawati E, Muladno. Effectiveness of ivermectin and albendazole against Haemonchus contortus in sheep in West Java, Indonesia. Trop Life Sci Res. 2016;27(1):135–44. Available from: https://pubmed.ncbi.nlm.nih.gov/27019686/
- Shahardar SRTRA, Wani IMAZA, Abbas M. Efficacy of ivermect, closantel, and fenbendazole against gastrointestinal nematodes of sheep in Kashmir valley. J Parasit Dis. 2017;41(2):380–2. Available from: https://doi.org/10.1007/s12639-016-0810-5
- Yuskiv ID, Tishyn OL, Yuskiv LL. Efficacy of levamisole against nematode infestations in dogs. Ukr J Vet Agric Sci. 2024;7(1):87–93. Available from: https://doi.org/10.32718/ujvas7-1.14
- Kudo N, Ishikawa N, Yamane A, Ikadai H, Oyamada T. Efficacy of levamisole alone and in combination with mebendazole against Gongylonema pulchrum infection in rabbits. J Vet Med Sci. 2015;77(1):113–6. Available from: https://doi.org/10.1292/jvms.14-0318
- Kudo N, Yoshioka T, Watanabe Y, Terazono Y, Takenouchi S. Reduced efficacy of ivermectin treatments in gastrointestinal nematode infections of grazing cattle in Japan. J Vet Med Sci. 2014;76(66):1487–91. Available from: https://doi.org/10.1292/jvms.14-0243
- Prangthip P, Tummatorn J, Adisakwattana P, Uthailak N, Tipthara P, Tarning J, et al. Anthelmintic efficacy evaluation and mechanism of N-methylbenzo[d]oxazol-2-amine. Sci Rep. 2023;13:1–13. Available from: https://doi.org/10.1038/s41598-023-50305-y
- Raza A, Rand J, Qamar AG, Jabbar A, Kopp S. Gastrointestinal parasites in shelter dogs: Shelter workers. Animals (Basel). 2018;8(7):108. Available from: https://doi.org/10.3390/ani8070108
- Soudkolaei AS, Kalidari GA, Borji H. Anthelmintic efficacy of fenbendazole and levamisole in native fowl in northern Iran. Parasit Vectors. 2021;14(1):104–12. Available from: https://doi.org/10.1186/s13071-021-04605-9
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
