Global Journal of Zoology
Grouping behavior of African forest elephants (Loxodonta cyclotis): A significant habitat exploitation strategy in Mount Cameroon National Park, Southwest region, Cameroon
Melle Ekane Maurice1*, Tadida Elvis Chembonui1, Kamah Pascal Bumtu1, Agbor James Ayamba1, Esther Eyong Mbi Arrabi2, Che Scholastica Nchang2, Aganya Benedatte Eyama2 and Tashe Vanesa Nwah3
2Department of Environmental Science, University of Buea, P.O. Box 63, Buea, Cameroon
3Department of Veterinary Medicine, University of Buea, P.O. Box 63, Buea, Cameroon
Cite this as
Maurice ME, Chembonui TE, Bumtu KP, Ayamba AJ, Mbi Arrabi EE, et al. (2023) Grouping behavior of African forest elephants (Loxodonta cyclotis): A significant habitat exploitation strategy in Mount Cameroon National Park, Southwest region, Cameroon. Glob J Zool 8(1): 006-014. DOI: 10.17352/gjz.000027Copyright
© 2023 Maurice ME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Wildlife grouping behavior is a significant survival strategy beneficial to all the group members. Migration to healthy food locations, defense from predators, mating, and social organization are some of the products of a well-established and organized grouping behavior of wildlife species such as elephants. Hence, the main objective of this study was to explore the grouping behavior of elephants on some ecological parameters in Mount Cameroon national park. Research data was collected within a period of four months by monitoring and observing elephant groups and their activities within their feeding ecology. Data collection was done during the first 15 days of each month and analyzed by Chi-square and correlation statistical models. In the study, elephant-group activity recorded a significance, X2 = 29.89 df = 8 p = 0.000, X2 = 12.95 df = 8 p < 0.05, and X2 = 11.801 df = 4 p = 0.019 on photo-period, atmospheric conditions, and habitat types respectively. The elephant groups also recorded a significant agreement, r = 0.061 p = 0.008, X2 = 17.35 df = 16 p < 0.05, and X2 = 27.62 df = 12 p = 0.006 on landscape, crop-farm, and crop-farm size estimate respectively. Additionally, elephant group activity recorded a significance, X2 = 18.39 df = 8 p = 0.018, r = 0.107 p < 0.05, X2 = 9.12 df = 8 p < 0.05, and X2 = 13.85 df = 8 p < 0.05 on farm destruction rate, farm destruction distance from human homes, elephant trails, and the crop-raided villages respectively. Group formation of elephants in the park is however reduced to smaller sizes probably due to the killing of elephants for human safety, a situation that could scare and cause some of the elephants to migrate to distant areas.
Introduction
The African elephant is the largest extant terrestrial mammal and is classified as a megaherbivore with a body size in excess of 1 ton [1]. Their large body size makes the scale of their impact on the ecosystem large and may result in changes to vegetation and biodiversity [2]. Elephants can thus be considered a keystone species [3,4], a term that is often extended to ‘ecological engineers’ [5]. This implies that their removal or significant increase in a system may have consequences for other components [6]. Many mammals including elephants, chimpanzees, cetaceans, and humans have variable social relationships involving high degrees of cooperative behavior [7-10]. These species live in flexible social groups where the size of the group and the relatedness of group members vary over time. This structure is referred to as fission-fusion because group structure is dynamic and group interactions are common. In some species, social structure within groups is more rigid than would be predicted by resource availability [11-13]. A single dominant individual, termed the leader, has significant influence over group behavior [7,10,14,15].
Leading characteristics such as age and size have been shown to influence dominance structures in socially complex species [16]. These individuals can influence many aspects of sociality including group movement, territory defense, and recognition [14,17]. For example, the order of individual animals in group movements has been studied in herbivores [14,15], focusing on leader identity. Dumont, et al. [14] found one animal dominated the first position in group movements and influenced the actions of the other group members in a group of grazing heifers, acting as the leader. In mammals that exhibit a social structure where dominance hierarchies exist, the position of the most dominant individual in the group can be assessed in differing social situations [15]. The order of individuals could vary based on social context, and the order has the potential to influence group interactions. In species where groups are sexually segregated and consist of related individuals, the female leader is referred to as the matriarch [7,18].
Group interactions are common in the fission-fusion social structure of African elephants, yet what mediates which groups are likely to fuse and when groups are likely to separate has not been fully explored. Because agonistic interactions in large, long-lived social mammals can incur heavy costs [12,13,19] and resource value is variable, agonistic interactions should be infrequent. Family groups from the same kinship group are more likely to exhibit resource-sharing, and less likely to behave agonistically towards each other [20,21]. Group interaction theory predicts that unrelated groups of equal ability are more likely to escalate agonistic interactions, whereas the subordinate group in interactions between unrelated groups of unequal ability will display submissive behavior [22]. Several alternative hypotheses are presented for what determines competitive quality in elephant groups. Group size may play an important role in determining the ability to defend the resource from an approaching group or to supplant an attendant group. I predicted that larger groups would be more likely to behave agonistically, and smaller groups would be more likely to behave in a submissive manner.
In highly social animals that spend their adult lives in groups of conspecifics, selective pressure exists to differentiate between group and non-group members, especially in situations where resources are limited or territories are maintained [9,12,23]. Interactions between groups vary from agonistic to affiliative based on the specific combination of ecological factors. Resource distribution influences group interactions such that species that use widely distributed resources are likely to display relatively few agonistic interactions in contrast to species that rely on patchily distributed resources [22,24]. In long-lived species where winner and loser effects are high (initial winners/losers tend to continue the same role), agonistic interactions are costly and therefore are rare [13,19]. In addition, affiliative group interactions may be beneficial, or even necessary in animal societies [25]. Hence, group formation is a trade-off between the costs of competing for resources and the benefits of cooperation [19].
Elephants have the potential to significantly alter their environment which can have far-reaching consequences, for example, their contributions can range from plant seed dispersal [26] to tree mortality [27], as well as increased availability of forage for other species [28-30], the excavation of waterholes in dry riverbeds [31], and the creation of refugia for invertebrates under toppled tree trunks [32]. An increase in elephant population size can amplify these impacts, especially in closed systems where spatial and temporal variation in impacts is reduced [2]. Therefore, any management decisions must take all possible ecosystem impacts into account.
Group formation in wildlife such as elephants is important in movement coordination, especially in new feeding areas where elephants would have to cover long distances to their new destinations. It is obvious that protected areas with huge elephant populations would result in the formation of huge groups. Elephant group formation and sizes in Mount Cameroon national park are comparatively smaller, which might be a result of poaching, and secondly, the montane vegetation difficult landscape structure, causing the elephants to alternatively migrate to distant areas with abundant vegetation to support their feeding. The elephant habitat in the national park is rich in vegetation but not easily accessible to the elephant population that may not be able to ascend most areas to feed due to their huge body sizes. Crop farms might have faced raiding problems from the elephants due to accessibility to the elephant population in the national park.
Materials and methods
Description of the study area
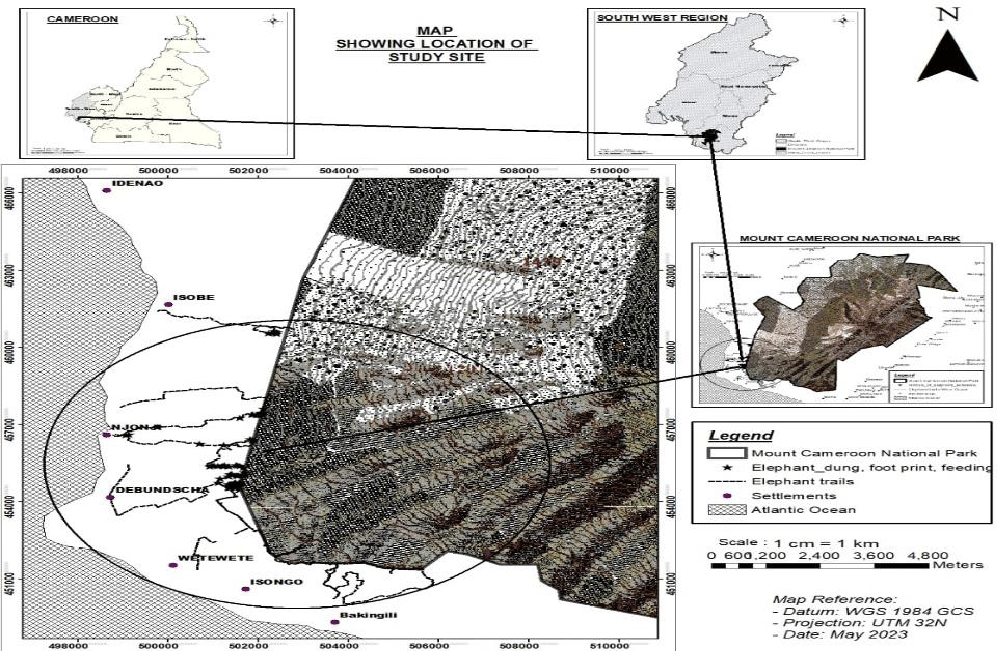
Mount Cameroon national park lies on the coast of the Gulf of Guinea, between latitude 3°57’ - 4°27’ N and longitude 8°58’-9°24’E (Figure 1). Climatically, the area is dominated by an equatorial climate of high rainfall and moderate tropical temperature. Average monthly temperatures are like any other part of the region, with the hottest month recording a monthly temperature of 33ºC (February-March) and the coldest months recording as low as 23 ºC (June–October) [33]. In the past, the rainy season occurred from March, extending to October, and the dry season from November to February each year. But due to the present climatic changes, the rainy season extends up to October and December. The biodiversity richness of Mount Cameroon national park area has been threatened over the years due to the rich volcanic soils which attract the development of agro-businesses. The region hosts the second wettest place on earth and has rich volcanic soil. Natural vegetation remains largely unbroken on its western slopes from sea level to the sub-alpine zone at the summit [34].
Mount Cameroon national park is home to a wide range of wildlife species such as drill (Papio leucophaeus), chimpanzee (Pan troglodytes), putty-nosed monkey (Cercopithecus nictitans), mona monkey (Cercopithecus mona), red-eared monkey (Cercopithecus erythrotis), red-cap mangabey (Cercocebus torquatus), Preuss“ guenon (Cercopithecus preussii) and crowned guenon monkey (Cercopithecus pogonias). However, the population of drills and chimps is fast dwindling due to hunting pressure and habitat loss. The forest elephant (Loxodonta africana) is one of the keystone species of the area. A survey carried out in 2003 indicates a population of 176 elephants in the national park [35].
Methods of data collection
The field research started with a pilot study to test the methods to be used for the research. The exercise witnessed adjustment of some variables on the eco-data sheet not feasible for data collection [36]. Hence, the real data collection program started in the month of February and ended in May. Three villages severely crop-raided by the elephants were chosen for research data collection and the crop farms which were raided were maize, cassava, banana, oil palm, and plantain. The elephants were monitored at a distance of about 100m during feeding periods and data was recorded. Data recording was also carried out on elephant trails, photo-period, habitat, landscape, crop destruction distance from human homes, human-elephant conflict villages, and the cropland destruction rate. The four months data collection method witnessed a two-week data collection program each month and was from 8:00 - 6:00 pm each day.
Data analysis
The research data was analyzed using SPSS version 25, and all the variables and sub-variables were tested against others by using exploratory and inferential statistics models such as chi-square (X2) and correlation. Ecological parameters such as photo-period, elephant trails, landscape, distance of crop destruction by the elephants from human residential houses, and the rate of cropland destruction were tested on the crop-raided activity of the elephants in the sample area.
Results
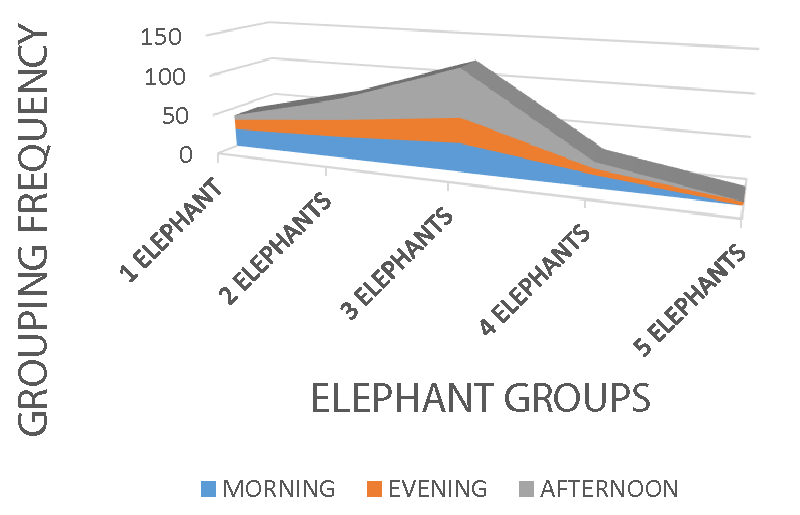
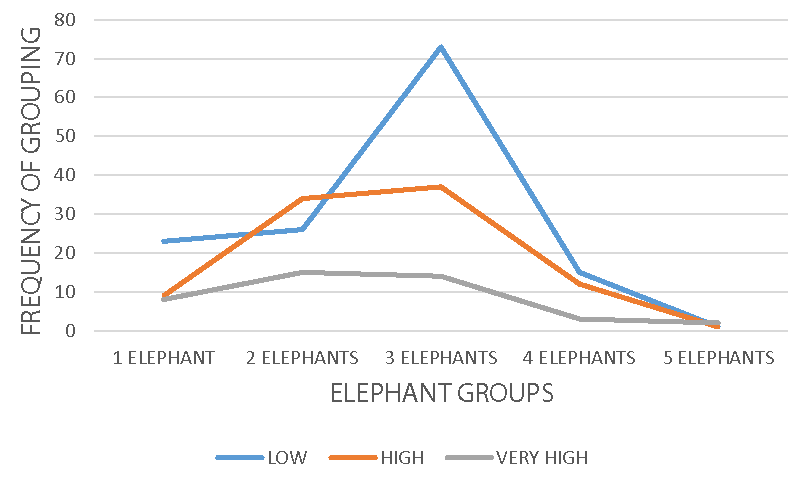
Elephant group activity recorded a significance, X2 = 29.89 df = 8 p = 0.000, X2 = 12.95 df = 8 p < 0.05, and X2 = 11.801 df = 4 p = 0.019 on photo-period (Figure 2), atmospheric conditions (Figure 3), and habitats (Figure 4) respectively. Group formation in the animal kingdom is very important, guaranteeing more safety against predators. Location of distance food sources, infant knowledge acquisition, movement coordination, and fighting rival groups are all related to the social organization and formation of a group. In wildlife, individuals outside the groups are not quite safe, the reason, sub-adult males or females leaves their maternal groups to join other groups for survival, protection, and avoidance of inbreeding. Group formation and management in African elephants is headed by an adult female, unlike other wildlife species such as big cats, bovids, and ungulates headed by an adult male. Feeding and food location identification knowledge in elephants is done by the group. Elephants are heavy feeders, with a consumption capacity of 140 kg - 150 kg a day, they can cover long distances for food location and only leaves when exhausted. In Mount Cameroon national park, elephants were observed highest in groups of three, while groups of five were the least. Small elephant groups are mostly observed in areas with food scarcity, especially during the dry season in the mosaic or savanna ecosystem, elephants have to cover very long distances for feeding. More so, migration behavior in elephants is food-related, hence movement from one area to another could be based on food search and protection.
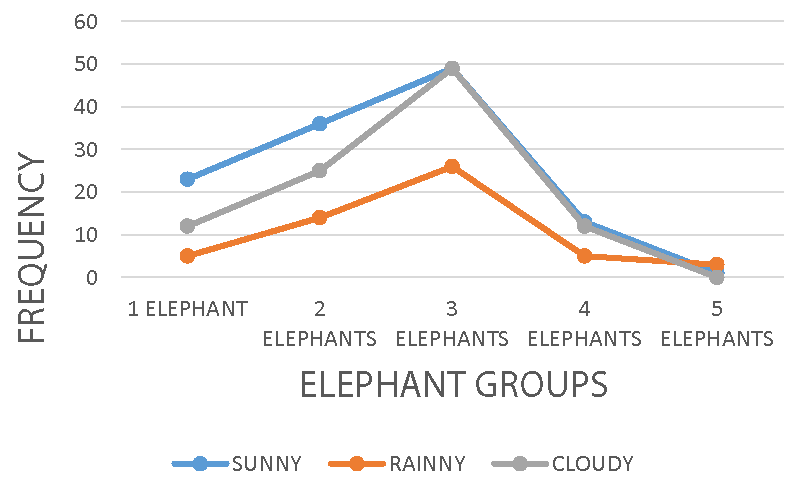
Group activity was highest during the morning and afternoon periods of the day and low in the evening periods. Groups of three elephants were observed with high activity rates within all the weather conditions, sunny, cloudy, and rainy respectively. Groups of two, four, and five elephants were the least spotted, meaning the elephant population must have suffered a reduction from poachers and some farmers for crop protection. Though the cost of killing an elephant is punishable by wildlife conservation law, farmers may kill them for their protection on the farms.
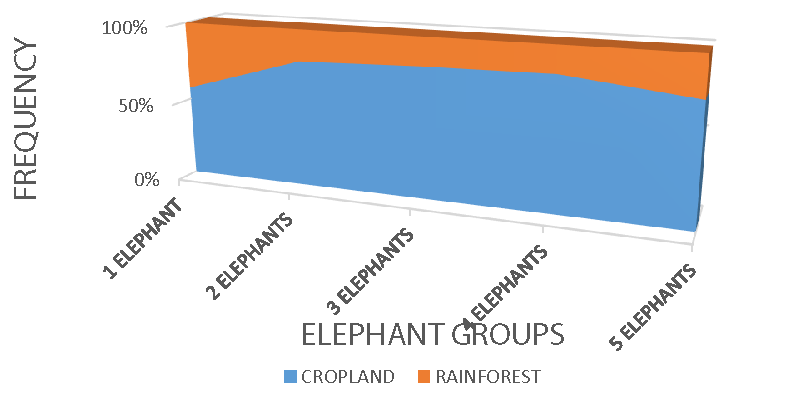
Elephants in Mount Cameroon national park seem to shift from rainforest habitat-feeding activity to crop-farm. The major reason might be an encroachment of human activity into the national park for cultivation, depleting the original habitat of elephants and other wildlife species. The transformation of elephant forest habitat to farmlands has created enormous problems for the management of the elephant population in the national park periphery. The study recorded the highest elephant feeding on cropland and the least on rainforest vegetation. Generally, the rainforest areas in Cameroon survive a huge population of elephants and other wildlife species, however, mount Cameroon national park’s periphery provides a more healthy feeding activity for elephants on cultivated land. Elephants’ dependence on cultivated land might be due to human encroachment, creating accessibility to crop feeding.
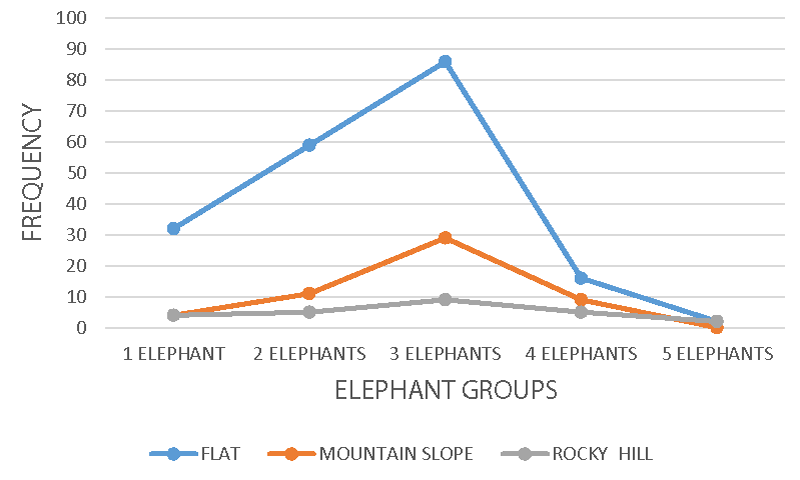
Furthermore, elephant group activity recorded a significant agreement, r = 0.061 p = 0.008, X2 = 17.35 df = 16 p < 0.05, and X2 = 27.62 df = 12 p = 0.006 on landscape (Figure 5), crop-farm (Figure 6), and crop-farm size estimate (Figure 7) respectively. The flat landscape at the periphery of the national park located at the foot of Mount Cameroon provides a comfortable dueling eco-zone for these huge body mammals, and access to montane forest vegetation might have been deliberately avoided due to ascension difficulties. The rocky and mountain slopes provided the least elephant group activity due to poor accessibility, believed to be caused by the elephant morphological structure, and the grassland vegetation that seems not to provide a healthy feeding to the elephants.
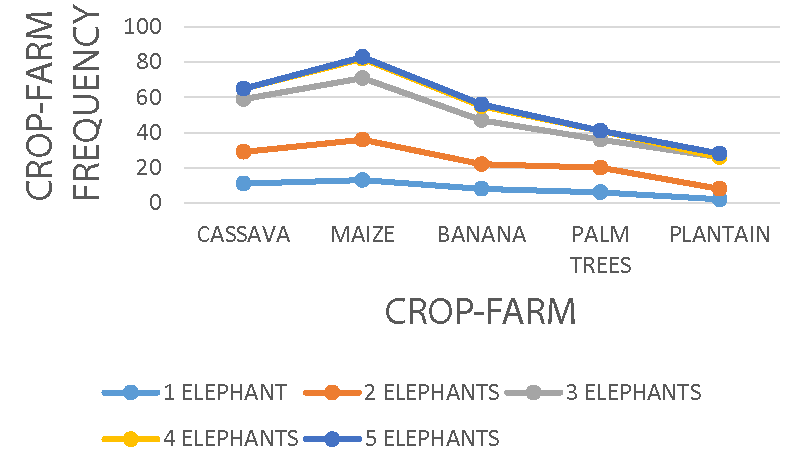
Crop farms with maize, cassava, and banana received the highest elephant group activity in the study, while oil palms and plantains recorded the least. Elephant groups do not strain to access crop farms, especially during the wet seasons during the peak of cultivation crop raiding was also more common. Commercial monoculture and peasant farming activity dominate the foot of Mount Cameroon where most of these elephants are found, providing a feeding leeway to the elephants that are believed to use the forest only for night rest.
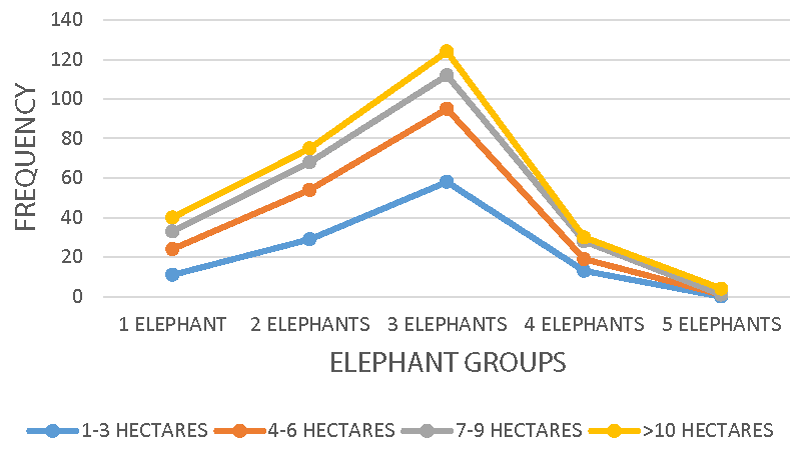
Crop farms of 4 - 6 hectares, 7 - 9 hectares, and above 10 hectares recorded the highest elephant-crop-destruction rate. Elephants seem to concentrate their feeding activity more on larger crop farms, especially those with Maize (Zea mays), banana (Musa acuminata), cassava (Manihot esculenta), oil palm (Elaeis guineensis) and plantains (Musa sapientum) with very nutritious vegetation. Some of these crops are cultivated seasonally, an attraction to more elephants during this period. The human population increase in the coastal region of Mount Cameroon for fishing and farming activities seems to be creating more conservation problems for national park management.
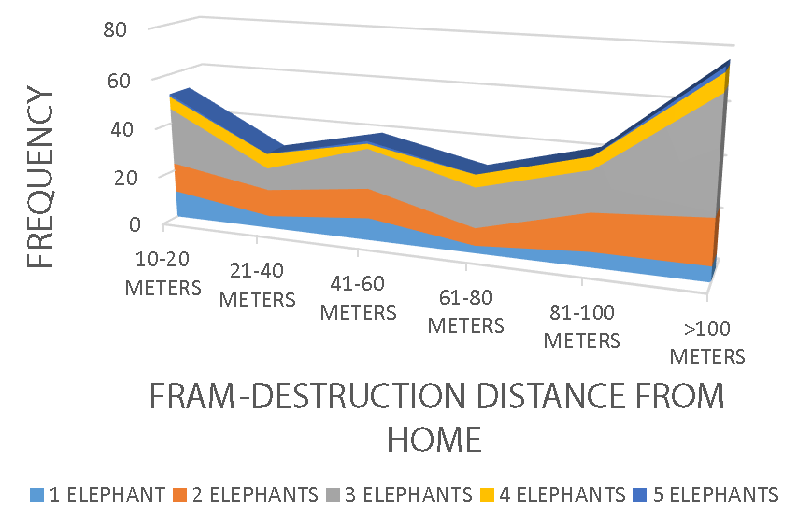
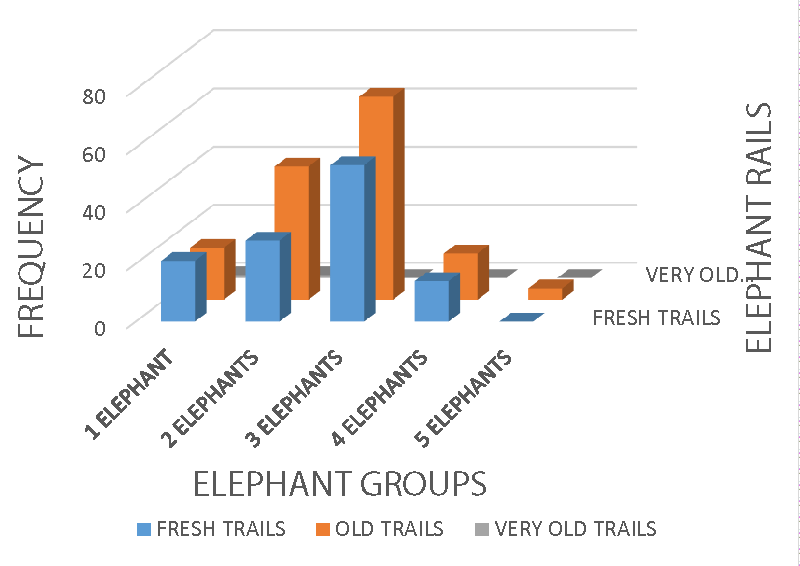
Additionally, elephant groups recorded a significance, X2 = 18.39 df = 8 p = 0.018, r = 0.107 p < 0.05, X2 = 9.12 df = 8 p < 0.05, and X2 = 13.85 df = 8 p < 0.05 on farm destruction rate (Figure 8), farm destruction distance from human homes (Figure 9), the trails (Figure 10), and the affected villages (Figure 11) respectively. Elephant encroachment into farmland is common in areas where villages are within the proximity of their forest habitat and feeding ranges, a conflict generation situation.
Resettlement of villages to distant locations free from elephant attacks has been the most appropriate solution to human-wildlife conflict. However, villages with huge human populations had often been a serious challenge to resettlement options due to the high cost of building houses for every household involved. On the west coast of Fako Division where the conflict is more dominantly frequent, the villages are populated and resettlement would be a remote option. Secondly, Limbe city is not really far from this area and most people preferably settle in some of the affected villages and travel to the city of Limbe every day for their commercial activities.
A correlation recorded on elephant groups shows clearly that the more people encroach into the national park, the more destruction by elephants. Though elephant poaching is rampant in Cameroon, human encroachment into the elephant habitat might be a key contributor to the destruction of crop farms, hence the conflict. More so, the killing of elephants at the foot of Mount Cameroon where the crop farms are concentrated might be for crop protection and not the tusk-trade money.
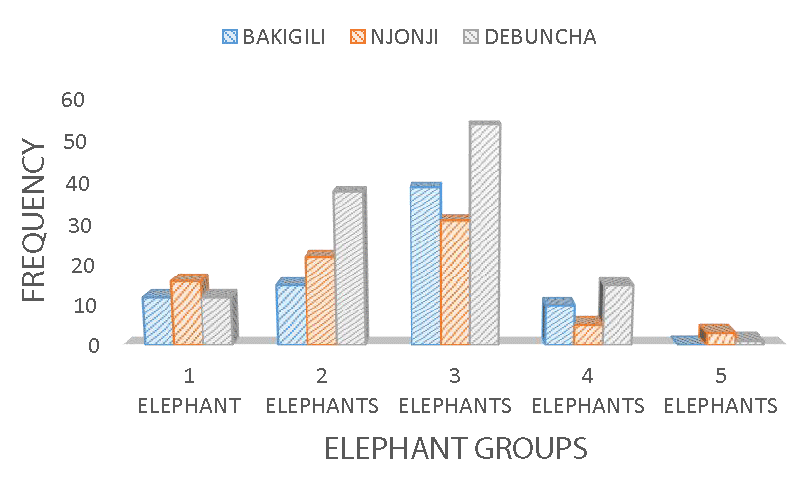
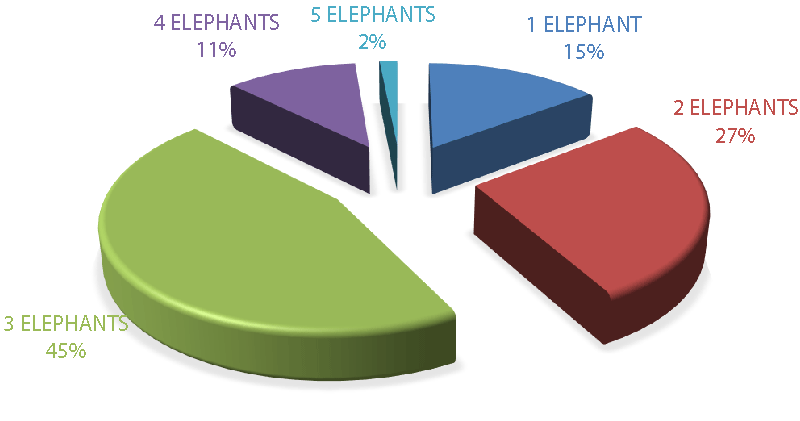
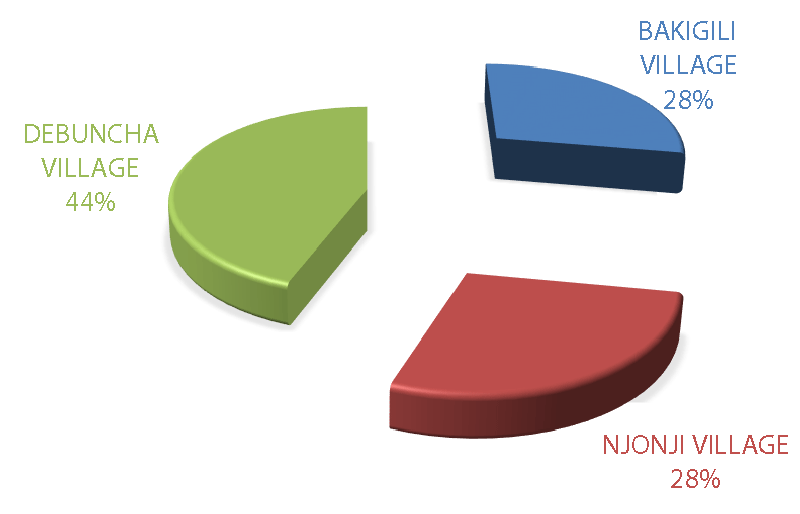
In rainforest-protected areas with a high elephant population, a herd can have a constitution of a hundred elephants, especially when there is less or no involvement in poaching activity. In this study, the biggest elephant herd/group constituted 5 - 10 elephants (2%) and was rarely observed compared to lower groups of 2 elephants (27%), 3 elephants (45%), and 4 elephants (11%) (Figure 12) respectively. Nonetheless, the highest number of elephant groups were observed in Debundcha village 44%, and was the furthest village from the city of Limbe among the sampled villages, compared to Njonji 28%, and Bakigili 28% (Figure 13) respectively.
Discussion
Resource distribution and competition over resources are considered the first order determinants of inter- and intra-group dominance structure in group-living mammals [11]. Social organization is generally structured by these and other ecological factors including predation risk [37]. In situations where resources are widely dispersed and not easily defendable, group dominance structure will be flexible and agonistic interactions rare [24]. Though this relationship was initially proposed for illustrating the effects of resource distribution on primate groups, it has since been applied to other mammals exhibiting similar social structures [10,38,39]. The social structure in these mammals is such that group membership is flexible, and groups exhibit frequent splitting and merging often based along lines of relatedness [20]. Dynamic group structures of this nature are referred to as fission fusion for that reason.
Flexibility in group membership of fission-fusion systems allows for the optimization of group characteristics such as group size, daily movements, and seasonal range based on resource availability and predation risk, which are also non-static properties of the ecosystem. However, the ability to optimize group characteristics based on socio-ecological factors may be variable between groups and will have associated fitness consequences. Group characteristics such as group number, territory quality, home range size, and characteristics of group leaders have been shown to influence direct or indirect fitness in a number of fission-fusion species [17,38,39]. The characteristics of leaders, especially age and size, dictate dominance rank in socially complex species [16,13]. Leaders can influence many aspects of sociality including group movement, territory defense, and recognition of other groups [14,17].
The reason why animals form herds cannot always be stated easily, since the underlying mechanisms are diverse and complex. Understanding the social behavior of animals and the formation of groups has been a fundamental goal in the field of sociobiology and behavioral ecology. The theoretical framework is focused on the costs and benefits associated with living in groups in terms of the fitness of each individual compared to living solitarily. Living in groups evolved independently multiple times in various taxa and can only occur if its benefits outweigh the costs within an evolutionary timescale. Thus, animals form groups whenever this increases their fitness compared to living in solitary [14].
Since foraging may be energetically costly (searching, hunting, handling, etc.) and may induce risk of predation, animals in groups may have an advantage, since their combined effort in locating and handling food will reduce the time needed to forage sufficiently. Thus, animals in groups may have shorter searching and handling times as well as an increased chance of finding (or monopolizing) highly profitable food, which makes foraging in groups beneficial for time minimizers and energy maximizers alike [40,41]. Staying together in groups often brings energetic advantages. Birds flying together in a flock use aerodynamic effects to reduce energetic costs, e.g. by positioning themselves in a V-shaped formation [42]. A similar effect can be observed when fish swim together in fixed formations. Another benefit of group living occurs when the climate is harsh and cold: By staying close together animals experience better thermoregulation because their overall surface-to-volume ratio is reduced. Consequently, maintaining adequate body temperatures becomes less energetically costly [40]. The collective force of a group mobbing predators can reduce the risk of predation significantly. Flocks of ravens are able to actively defend themselves against eagles and baboons collectively mob lions, which is impossible for individuals alone. This behavior may be based on reciprocal altruism, meaning animals are more likely to help each other if their conspecifics did so earlier [40]. Animals living in groups are more likely to find mates than those living in solitary and are also able to compare potential partners in order to optimize the genetic quality of their offspring [40].
Social structure and organization, which include the patterning of relationships and the system of interactions between individuals, may affect foraging, reproductive opportunities, antipredatory benefits, vulnerability to disease, and information transfer [38,43-49], making them important in the study of animal species. Social organization is thought to evolve in response to resource-risk distributions, with female social communities or groups being formed primarily in response to predation, limited food resources (such that being part of a group helps with access to food through between-group competition), and/or infanticide avoidance [11,24,37,50-52].
Depending on the spatial distribution, quantity, and quality of resources, competition regimes vary [53], resulting in social structures with different extents of inter- and intragroup competition and group sizes [11,22,24,37,50,54-59]. While there are various advantages of group living, within-group feeding competition is thought to be the primary cost of group living, with an increase in group size necessitating an increase in the group’s daily travel in order to meet food requirements [22,50,54,58].
Asian and African savannah elephants form matriarchal societies, with females and their dependent offspring living together in groups, and adolescent males dispersing from the groups and being largely solitary thereafter [7,8,60-62]. However, based on previous studies, there seemed to be differences in social structure within and between elephant species, possibly stemming from different sampling methods and ecology. Mother–offspring units and “family groups” that referred to one to a few closely related mother-offspring units [63-65] were at the base of the hierarchical female organization of African savannah elephants. In Amboseli, family groups identified at the beginning of the study were later called core groups, and associations of family or core groups were termed bond groups [8,12].
Conclusion
The social organization of African elephants is a constitution of herds or groups uniquely made up of family members: adult females and males, sub-adult males, and females and juveniles. Elephant groups or herds are very important to the ecological survival of elephants, hence, feeding and location of food areas, protection, and procreation are the responsibilities of the herd/group members. Protected areas with large elephant populations constitute herds with huge numbers of elephants. In Mount Cameroon national park, the elephant herds or groups are small since the highest number observed was between 5 and 10 elephants. Though elephant poaching in this national park is not common, the elephant population seems to reduce probably due to migration to distant feeding sites towards Ndian. However, human-elephant conflict on cropland is severe at the buffer zone or periphery of the national park resulting in human and elephant life loss. Smaller elephant groups or herds were more frequently met on cropland, destructively feeding on crops, a situation that has made farmers to suffer lower annual yields. Elephant killing in this area is believed to be for crop protection and not for the commercial tusk trade which has caused elephant population reduction in most national parks in Cameroon and beyond.
- Owen-Smith RN. Megaherbivores. The influence of very large body size on ecology. Cambridge University Press, Cambridge. 1988.
- Kerley GIH, Landman M, Kruger L, Owen-Smith N, Balfour D, Boer WFD, Gaylard A, Lindsay K, Slotow R. Effects of elephants on ecosystems and biodiversity. In R. J. Scholes and K. G.Mennell (Eds.). Elephant management; a scientific assessment for South Africa. Wits University Press, Johannesburg, South Africa. 2008; 146–205.
- Power ME, Tilman D, Estes JA, Menge BA, Bond WJ, Mills LS, Daily G, Castilla JC, J. Lubchenco, and R. T.Paine. (1996). Challenges in the quest for keystones. BioScience 46:609–620
- Sinclair AR. Mammal population regulation, keystone processes and ecosystem dynamics. Philos Trans R Soc Lond B Biol Sci. 2003 Oct 29;358(1438):1729-40. doi: 10.1098/rstb.2003.1359. PMID: 14561329; PMCID: PMC1693264.
- Jones CG, Lawton JH, Shachak M. Organisms as ecosystem engineers. Oikos. 1994; 69:373–386.
- Mills LS, Soule ME, Doak DF. The keystone-species concept in ecology and conservation: Management and policy must explicitly consider the complexity of interactions in natural systems. BioScience. 1993; 43:219–224.
- Douglas-Hamilton I. On the ecology and behavior of the African elephant. Ph.D. thesis, University of Oxford. 1972.
- Moss CJ, Poole JH. Relationships and social structure of African elephants. In: Primate Social Relationships: An Integrated Approach (Ed. by Robert A. Hinde). Sunderland, MA: Sinauer Associates. 1983; 315-325.
- Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge: Harvard University Press. 1986.
- Christal J, Whitehead H. Social affiliations within sperm whales (Physeter macrocephalus) groups. Ethology. 2001; 107:323-340.
- Sterck EHM, Watts DP, van Schaik CP. The evolution of female social relationships in nonhuman primates. Behavioral Ecology and Sociobiology. 1997; 41:291-309.
- Archie EA, Morrison TA, Foley CA, Moss CJ, Alberts SC. Dominance rank relationships among wild female African Elephants, (Loxodonta Africana). Animal Behaviour. 2006a; 71:117-127.
- Wittemyer G, Getz WM. Hierarchical Dominance Structure and Social organization in African elephants, Loxodonta Africana. Animal Behavior. 2007; 73:671-681.
- Dumont B, Boissy A, Achard C, Sibbald AM, Erhard HW. Consistency of animal order in spontaneous group movements allows the measurement of leadership in a group of grazing heifers. Applied Animal Behaviour Science. 2005; 95:55-66.
- Fischoff IR, Sundaresan SR, Cordingley J, Larkin HM, Sellier MJ, Rubenstein DI. Social relationships and reproductive state influence Leadership roles in movements of plains zebra, Equus burchellii. Animal Behaviour. 2007; 73:825-831.58
- Robbins MM, Robbins AM, Gerald-Steklis N, Steklis HD. Long-term dominance relationships in female mountain gorillas: strength, stability and determinants of rank. Behavior. 2005; 142:779-809.
- McComb K, Moss C, Durant SM, Baker L, Sayialel S. Matriarchs as repositories of social knowledge in African elephants. Science. 2001 Apr 20;292(5516):491-4. doi: 10.1126/science.1057895. PMID: 11313492.
- Krebs CJ, Davies NB. Behavioral ecology: an evolutionary approach, 4th ed. Blackwell, Malden, Ma. 1997.
- Crowley PH. Dangerous games and the emergence of social structure: evolving memory-based strategies for the generalized Hawke-dove game. Behavioral Ecology. 2001; 12:753-760.
- Archie EA, Moss CJ, Alberts SC. The ties that bind: genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proc Biol Sci. 2006 Mar 7;273(1586):513-22. doi: 10.1098/rspb.2005.3361. PMID: 16537121; PMCID: PMC1560064.
- Whitehouse AM, Hall-Martin AJ, Knight MH. A comparison of methods used to count the elephant population of the Addo Elephant National Park, South Africa. African Journal of Ecology. 2001; 39:140-145.
- Wrangham RW, Conklin NL, Etot G, Obua J, Hunt KD, Hauser MD, Clark AP. The value of figs to chimpanzees. International Journal of Primatology. 1993; 14:243-256.
- Spong G, Creel S. Effects of kinship on territorial conflicts among groups of lions, Panthera leo. Behavioral Ecology and Sociobiology. 2004; 55:325-331
- Isbell LA, Young TP. Ecological models of female social relationships in primates: similarities, disparities, and some directions for future clarity. Behavior. 2002; 139:177-202.
- Lazaro-Perea C. Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defense and assessment of neighbors. Animal Behaviour. 2001; 62:11-21.
- Kerley GIH, Landman M. The impacts of elephants on biodiversity in the Eastern Cape Subtropical Thickets. South African Journal of Science. 2006; 102:395-402.
- Landman M, Kerley GIH, Schoeman DS. Relevance of elephant herbivory as a threat to Important Plants in the Addo Elephant National Park, South Africa. Journal of Zoology. 2008; 274: 51-58.
- Smallie JJ, O'Connor TG. Elephant utilization of Colophospermum mopane: possible benefits of hedging. Afr J Ecol. 2000; 38:352-359.
- Skarpe C, Aarrestad PA, Andreassen HP, Dhillion SS, Dimakatso T, du Toit JT, Halley DJ, Hytteborn H, Makhabu S, Mari M, Marokane W, Masunga G, Modise D, Moe SR, Mojaphoko R, Mosugelo D, Mptsumi S, Neo-Mahupeleng G, Ramotadima M, Rutina L, Sechele L, Sejoe TB, Stokke S, Swenson JE, Taolo C, Vandewalle M, Wegge P. The Return of the Giants: Ecological effects of an increasing elephant population. Ambio. 2004; 33:276-282.
- Makhabu SW, Skarpe C, Hytteborn H. Elephant impact on shoot distribution on trees and on re-browsing by smaller browsers. Acta Oecol. 2006; 30:136-146.
- Conybeare A, Haynes G. Observations on elephant mortality and bones in water holes. Quaternary Research. 1984; 22:189-200.
- Govender N. The effect of habitat alteration by elephants on invertebrate diversity in two small reserves in South Africa. MSc Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa. 2005.
- Limbe City Council (2014) The Limbe City Council Report.
- Cheek M, Cable S, Hepper FN, Ndam N, Watts J. Mapping plant biodiversity on Mount Cameroon. In. Van der Maasen LJG, van der Burgt XM, van Medenbach de Rooy JM. (Eds.). The biodiversity of African plants. Proceedings XIVth AETFAT Congress, Wageningen, The Netherlands. Kluwer Academic Press, Dordrecht. 1994; 110-210.
- Ekobo A. Elephant monitoring in the Mount Cameroon region, Report to WWF. 2003.
- Maurice ME, Blessing NT, Veronique M, Ebong EL, Moumine NA. Organic Waste-Dumps: A Food Subsidy to the Wild-City Birds in Limbe, Southwest Region, Cameroon. Environ Pollut Climate Change. 2022; 6:283.
- Wrangham RW. An ecological model of female-bonded primate groups. Behavior. 1980; 75: 262-300.
- Wittemyer G, Getz WM, Vollrath F, Douglas-Hamilton I. Social dominance, seasonal movements, and spatial segregation in African elephants: a contribution to conservation behavior. Behavioral Ecology and Sociobiology. 2007; 61:1919-1931.
- Popa-lisseanu AG, Bontadina F, Mora O, Ibanez C. Highly structured fission-fusion societies in an aerial-hawking, carnivorous bat. Animal Behaviour. 2008; 75:471-482.
- Majolo B, Huang P. Group living. In J Vonk & T Shackelford (Eds.), Encyclopedia of Animal Cognition and Behavior. 2020.
- Pyke GH, Pulliam HR, Charnov EL. Optimal foraging: a selective review of theory and tests. Quarterly Review of Biology. 1977; 52:137-154.
- Portugal SJ, Hubel TY, Fritz J, Heese S, Trobe D, Voelkl B, Hailes S, Wilson AM, Usherwood JR. Upwash exploitation and downwash avoidance by flap phasing in ibis formation flight. Nature. 2014 Jan 16;505(7483):399-402. doi: 10.1038/nature12939. PMID: 24429637.
- Gompper ME. Sociality and asociality in white-nosed coatis (Nasua narica): foraging costs and benefits. Behavioral Ecology. 1996; 7:254-263.
- Hass CC, Valenzuela D. Anti-predator benefits of group living in white-nosed coatis (Nasua narica). Behav Ecol Sociobiol. 2022; 51:570-578.
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005 Apr 29;308(5722):648-52. doi: 10.1126/science.1106477. PMID: 15860617.
- Silk JB. The adaptive value of sociality in mammalian groups. Philos Trans R Soc Lond B Biol Sci. 2007 Apr 29;362(1480):539-59. doi: 10.1098/rstb.2006.1994. PMID: 17363359; PMCID: PMC2346516.
- Voelkl B, Noë R. Simulation of information propagation in real-life primate networks: longevity, fecundity, fidelity. Behav Ecol Sociobiol. 2010; 64:1449-1459.
- Langwig KE, Frick WF, Bried JT, Hicks AC, Kunz TH, Kilpatrick AM. Sociality, density-dependence and microclimates determine the persistence of populations suffering from a novel fungal disease, white-nose syndrome. Ecol Lett. 2012 Sep;15(9):1050-7. doi: 10.1111/j.1461-0248.2012.01829.x. Epub 2012 Jul 2. PMID: 22747672.
- Vander Waal KL, Atwill ER, Isbell LA, McCowan B. Linking social and pathogen transmission networks using microbial genetics in giraffe (Giraffa camelopardalis). J Anim Ecol. 2014; 83:406-414.
- Jarman PJ. The social organization of antelope in relation to their ecology. Behavior. 1974; 48(3-4):215-267. https://doi.org/10.1163/156853974X00345
- Clutton-Brock TH, Harvey PH. Primate ecology, and social organization. J Zool. 1977; 83(1):1-39.
- Chapman CA, Chapman LJ, Wrangham RW. Ecological constraints on group size: an analysis of spider monkey and chimpanzee subgroups. Behav Ecol Sociobiol. 1995; 36:59-70.
- Nicholson AJ. An outline of the dynamics of animal populations. Aust J Zool. 1954; 2:9-65.
- Terborgh J, Janson CH. The socioecology of primate groups. Annual Review of Ecology and Systematics. 1986; 17: 111-136.
- Dunbar RIM. Primate social systems, New York Cornell University Press. 1988.
- van Schaik CP, van Hooff JARA. On the ultimate causes of primate social systems. Behaviour. 1983; 85: 91-117.
- Isbell LA. Contest and scramble competition: patterns of female aggression and ranging behavior among primates, Behav Ecol. 1991; 2:143-155.
- Janson CH, Goldsmith ML. Predicting group size in primates: foraging costs and predation risks. Behav Ecol. 1995; 6:326-336.
- Wittiger L, Boesch C. Female gregariousness in western chimpanzees (Pan troglodytes verus) is influenced by resource aggregation and the number of females in estrus. Behavioral Ecology and Sociobiology. 2013; 67:1097-1111.
- McKay GM. Behavior and ecology of the Asiatic elephant in southeastern Ceylon. Smithsonian Contributions to Zoology. 1973; 125:1-113.
- Sukumar R. The Asian elephant: ecology and management. Cambridge: Cambridge University Press. 1989.
- Vidya TNC, Sukumar R. Social organization of the Asian elephant (Elephas maximus) in southern India inferred from microsatellite DNA data. Journal of Ethology. 2005; 23: 205-210.
- Buss IO, Smith NS. Observations on reproduction and breeding behavior of the African elephant. Journal of Wildlife Management. 1966; 30:375-388.
- Paley RGT, Kerley GIH. The winter diet of elephant in Eastern Cape Cape Subtropical Thicket, Addo Elephant National Park. 1998.
- Kerley GIH, Landman M, Kruger L, Owen-Smith N, Balfour D, De Boer WF, Gaylard A, Lindsay K, Slotow R. Effects of elephants on ecosystems and biodiversity.In: R.J. Scholes & K.G. Mennell (Eds), Assessment of Duffy, et al. Movement patterns of African elephants in different habitat types 27 South African elephant management Witwatersrand University Press, Johannesburg. 2008; 146-205.
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
