Promising role of Carob (Ceratonia siliqua L) phytochemical components against neurotoxicity induced by monosodium glutamate
Zoology Department, Faculty of Science, Mansoura Univ, Egypt
Author and article information
Cite this as
IH El-Sayyad H, Elkholy WME, Hamed WAE (2017) Promising role of Carob (Ceratonia siliqua L) phytochemical components against neurotoxicity induced by monosodium glutamate. Glob J Zool. 2017; 2(1): 024-032. Available from: 10.17352/gjz.000008
Copyright License
© 2017 IH El-Sayyad H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The phytochemical constituents of Carob (Ceratonia siliqua L) showed therapeutic medical importance especially concerning neurotoxicity which represents the major public health problem. Neurodegenerative disorders are developed from different metabolic diseases and chemical component such as monosodium glutamate. It is the widely used chense sodium salt of the non-essential glutamic amino acid. It is one of the most popular fl avor enhancer. Monosodium glutamate is excitatory neurotransmitter in brain, increased the perception of wetness and saltiness as a taste sensation UMAMI. Treatment with the carob extract or their phytochemical constituents either protect or ameliorates these diseases which is promising.
Phytotherapy is of medical importance due to the side effects of pharmaceutical drugs. Knowing the phytochemical components of plants and pharmacologic action of each constituents and standardization procedures for use and its clinical effective. Neurodegeneration is the progressive breakdown of neurons leading to neurological disorder such as Parkinson’s and Alzheimer’s, and Huntington’s diseases [1]. Monosodium glutamate (MSG) is excitatory neurotransmitter in brain, mediating fast synaptic transmission and increased the perception of wetness and saltiness as a taste sensation UNAMI [2]. It is a water soluble bad chemical component showing a potent UNAMI comes from the fifth taste receptors on the tongue which are quite different from that of the brain [3]. This led individual to consume a large amount of food due to its characteristic flavor enhancer which alter physiological processes, especially the functioning of the nervous system [4,5].
The glutamate is produced by the brain and serves as a as a nerve impulse transmitter in the brain to manage the functional activity of body organs [6]. The brain generates its n glutamate with an intricate own transport system to protect the brain cells. The excess circulating glutamate derived from the food material is kept separated from the glutamate inside the brain. The shifted glutamate concentration being increased in the intracellular region and low in the extracellular ones. This may exert strokes and damage the blood-brain barrier [7,8].
Administration of glutamate to experimental animals and humans [9,10], led to the development of depression and anxiety in the form of imbalance of mood and emotions, abnormalities of limbic system structures [11], and disruption of the hypothalamic pituitary adrenal axis [12]. Glutamate was linked to many diseases such as Alzheimer’s disease, Huntington’s disease and Wernicke’s encephalopathy [13,14], as well as retinal ischemia leading to loss of ganglion cells [15]. In vitro studies of MSG (20 mM) on astrocyte culture cells revealed increased a liberation of reactive oxygen species and apoptotic cell death [16].
The present review aimed to illustrate the neurologic disorders induced by monosodium glutamate and the promising role of carob extracts or their phytochemical constituents in treating or protecting the brain disorders.
Carob (Ceratonia siliqua L)
Carob fruit of Ceratonia siliqua L. is belong to Leguminosae family. It is widely cultivated in the Mediterranean region for ornamental and industrial purposes. Carob fruit is dark-brown with an elongated or curved shape and composed of two main components: the pulp (90%) and the seeds (10%). Its seeds contain approximately 90% galactomannans and used in food industry [17]. Papagiannopoulos et al. [18], reported that the carob pods contain 448 mg/kg polyphenols including gallic acid, hydrolyzable and condensed tannins, flavonol-glycosides, and traces of isoflavonoids. Carob powder contained Eleven phenolic compounds such as pyrogallol, catechol, chlorogenic and protocatechuic, coumarin, cinnamic, ferulic, gallic acid and vanillic are detected [19].
Sucrose represents about 70% of the carob pulp and composed mainly of fructose and glucose [20]. Protein is reached to about 7.6% and fat content is about 0.2 – 2.3% [21]. Fiber, cyclitols, polyphenols and tannins are the main constituents of carob fruits which possess anti-cancer, anti-diabetes, anti-diarrheal and anti-hyperlipidemic activity [22,23]. Also, carob is rich in micronutrients like amino acids including aspartic acid, glutamic acid, serine, glycine, histidine, arginine, threonine, alanine, tyrosine, valine, proline, methionine, isoleucine, leucine, cysteine, phenylalanine and lysine [24].
The carob powder is characterized by its natural sweetener with flavor and free from caffeine and theobromine which makes it suitable to be used in Europe instead of chocolate. It is widely used as a cocoa substitute in baking, cereal bars, ice creams and light products as CarovitTM (Alimcarat S.L., Spain) [25], as well as in cereal-derived foods for celiac people [26].
The pod and leaves of Ceratonia siliqua (carob) are rich in active compounds such as peripheral benzodiazepine receptor widely used as chemopreventive agents [27]. Its fruit is rich in flavonoids and condensed tannins [28], as well as contained of about 96.5% of protein and rich in glutamic acid, aspartic acid and arginine [29].The carob pods is a rich source of polyphenol reaching approximately 80% with high antioxidant activity [20,23,30]. Also, it is rich in linoleic and alpha-linolenic acid [31]. The antioxidant of ethyl acetate extracts of carob tree leaves scavenge 1,1-diphenyl-2-picrylhydrazyl liberated radicals than the diethyl ether and dichloromethane extracts [32].
Aqueous extract of carob (Ceratonia siliqua L.) (600 mg/kg body weight) pods protected against ethanol (6 g/kg bw)-induced hepatotoxicity characterized by elevated hepatic aspartate aminotransferase and alanine aminotransferase, lipid peroxidation and depletion of the antioxidant enzymes [33].
Ceratonia siliqua extracts, showed a potential DNA damage of murine leukaemia cells L1210 as well as protect against oxidative stress of H2O2 [34].
Monosodium glutamate related neurotoxicity
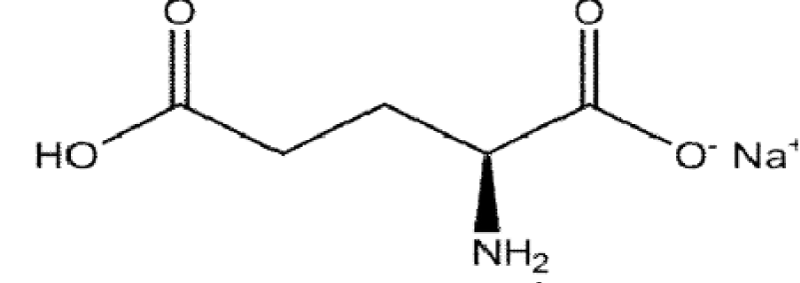
Monosodium glutamate (MSG) is the sodium salt of the glutamic acid. It is a non-essential amino acid, having unique flavor-enhancing widely used as a food additive (Figure 1).
Glutamic acid is abundant in protein rich food stuffs such as milk, meat, fish, cheese, tomato products, and soy sauces. It is one of the most popular flavor enhancer. It is excitatory neurotransmitter in brain, mediating fast synaptic transmission in one third of all CNS synapses. It increased the perception of wetness and saltiness as a taste sensation UMAMI and is used in many commercial packed food (Maggi Noddles, Knorr Soup etc), restaurant and household cooking. It is a natural components of many fermented or aged foods, such assoy sauce, fermented bean paste, and cheese, and is also in yeast extract [1]. In European countries, the intake of glutamate from food ranged from 5 to 12 g/day compared to Asian countries which reached from 1.2 to 1.7 g/day [35].
Administration of glutamate disrupted the biological function in experimental animals and humans causing depression and anxiety which are characterized by imbalance of mood and emotions, abnormalities of limbic system structures, associated by reduction in monoaminergic signaling, with depletion of serotonin (5-hydroxytryptamine, 5-HT) (Meyer et al. 2006).
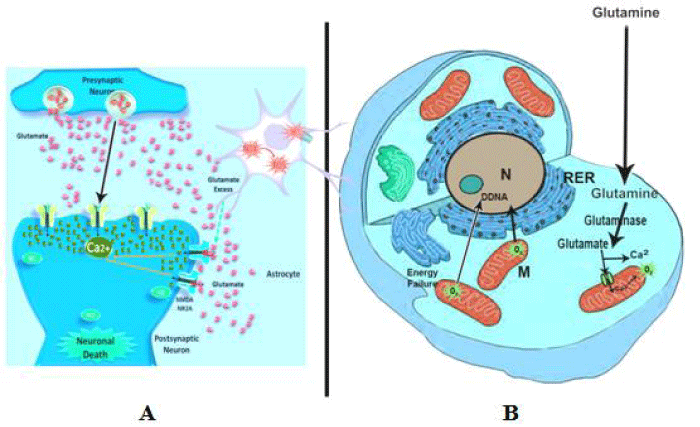
Glutamate-induced neurotoxicity characterized by neuronal damage [36,37], via intracellular increase of Ca2C levels through increase liberation of the N-methyl-D-aspartate receptors [38], or enhancement secretion of glutaminase utilizing glutamate as a substrate [39].
Glutamate released its signal through ionotropic and metabotropic glutamate receptors. Ionotropic receptors promote the ion channel pore that activates when glutamate binds to the receptor meanwhile metabotropic receptors enhanced ion channels on the cell membrane via a signaling cascade that form G-protein-coupled receptors primarily on neurons and glial cells [40,41]. Ionotropic receptors divided into four subtypes depending on their ligand binding properties such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA receptors), kainite receptors, N-methyl-D-aspartate receptor (NMDA receptors) and delta receptors, They promote the excitatory synaptic transmission in the central nervous system for synaptic plasticity, which is important for learning and memory [42]. Thee synaptic receptors located primarily on the membranes of neuronal cells. The interactions of glutamate with its ionotropic NMDA receptors led to neurotoxic changes due to the release excessive amounts of calcium to enter the neuron [43,44] and consequently contributed to the development of neurodegenerative disorders [45-47].
The receptor AMPA located in the different parts of the brain mediating the synaptic transmission which categorized into four subunits including GluR1, GluR2, GluR3 and GluR4. Its dimerization start in the endoplasmic reticulum [48].
The second type is kinate receptors. They have both presynaptic and postsynaptic actions with a limited distribution in the brain comparing to the other types of ionotropic receptors. Kainic acid induced seizures, through the activation of kainate receptors containing both GluK2 subunit and AMPA receptors [49], N-methyl-D-aspartate receptor is a glutamate receptor and ion channel protein found in brain cells. It is activated when glutamate and glycine (or D-serine) bind together and activated for permeability of positively charged ions to pass through the cell membrane. The NMDA receptor is responsible for controlling synaptic plasticity and memory function [50].
Ionotropic glutamate delta 2 receptor is a protein that encoded in the human GRID2 gene. The receptors are expressed mainly in Purkinje cells in the cerebellum [51], and promote the synaptogenesis, synaptic plasticity, and motor coordination [52].
Glutamate is a naturally occurring amino acids reaching to 4 to 15% of all amino acids [53], and its level in the brain attained 104 μM. It is synthesized by neurons and not cross the blood-brain barrier [54]. It is responsible for maintaining developmental plasticity and memory [55]. However, intake diet containg MSG is followed by an increase in plasma glutamate level for 1 to 3 hours [56]. The first step of excitotoxicity is the release of Mg(2+) from mitochondria to the cytosol and accumulation of Ca(2+) [57]. Also, glutamate is then converted into glutamine in astrocyte through a glutamine-reuptake system and loss its function [58]. Most of the excitatory neurons in the brain are glutamatergic; moreover, it is found that many of the nerve-endings release glutamate. Presynaptic depolarization maintain vesicles to liberate glutamate into the synapses through exocytosis, which consequently bind to the post-synaptic ionotropic receptors and depolarize the synaptic cell. Neuronal cell death is come after the liberation of glutamate into the synaptic space which stimulates glutamate receptors of the NMDA subtype, leading to an influx of calcium and sodium and depolarization of the postsynaptic neuron. NMDA receptors become inactive for glutamate transport into cells. Neuronal ischemia impaired cell respiration through depletion of ATP coincides with failure of glutamate transport and neuronal depolarization. These led to activate NMDA R causing release of calcium, mitochondrial dysfunction and production of reactive oxygen species the cause of inflammation and neuronal cell death [59,60] (Figure 2).
Glutamate is linked to many neurological diseases such as Alzheimer’s disease, Huntington’s disease, glaucoma and Wernicke’s encephalopathy [61]. Also, it is associated with “kindling” limbic seizures in hippocampus of rodent and cerebral cortex of patients via repeated electrical stimulation dependent on the activation of N-methyl-D-aspartate (NMDA) receptors. Microdialysis increased the extracellular concentration of glutamate and aspartate before or during seizure onset, as a result of either enhanced amino acid release or impaired uptake contributed to seizure occur [60].
In vitro studies revealed that the excitatory amino acids such as L-aspartate and L-glutamate and micromolar kainic acid induced massive shedding of the rod photoreceptor disc and loose the contact with the pigment epithelium in eye cups of Xenopus laevis [62]. In postnatal rodents, daily injection of MSG led to impairment of vision via increase of retinal lesions and optic nerve degeneration [63]. Acute retinal ischemia was incorporated in retinal damage associated with accumulation of glutamate in aqueous humor [64] leading to retinal detachment in patients [65], and increase retinal damage in neonatal rats [66].
Intra-vitreal injections of glutamate induced overexpression of the excitatory amino acid transporter 1 (EAAT-1), thinning of retina and reduction of ganglion and increase glial fibrillary acidic protein (GFAP) in Müller cells, CD11b in microglia, and iNOS and GRP78 in glial cells [67]. MSG-treatment induced a marked increase in β-amyloid in the hippocampus by >4-fold and >5-fold after 10 days in both oral and subcutaneous administration [68].
Monosodium glutamate have the great ability to penetrate the placental barrier and accumulate in the embryonic tissues especially in the brain tissue of fetal mice [69], and cause acute necrosis of the acetylcholinesterase-positive neurons in the area postrema in the mother rats and their fetuses [70]. Monosodium glutamate (4mg/g body weight)- treatment decreased the antioxidant capacity of midbrain region and increased lipid peroxidation [71], via increasing thiobarbituric acid reactive substances and delay the acrophases of GSH and catalase as a result of increased glutamate levels [72].
Also, it led to an increase of neuronal cell death of the medial basal hypothalamic (arcuate nucleus) neurons of neonatal mice associated with overexpression of the N-methyl-d-aspartate glutamate receptor subunit of the damaged neurons [73].
Monosodium glutamate and amyloid deposition
Monosodium glutamate (MSG) is the main cause of excitotoxicity associated with brain disorders including brain ischemia and neurodegenerative disorders [74]. In Alzheimer’s disease temporal cortex, there was a marked decrease of glutamate uptake comparing with non-changed NMDA receptor [75]. Its treatment led to stimulation of neurotransmitters and consequently nitric oxide (NO)-mediated neurotransmission pathway as a result of increased NO synthase produced from the arginine. Increased release of NO in endothelial cells vasodilated neighboring vascular smooth muscle cells and consequently induce Chinese restaurant syndrome and/or glutamate-induced asthma, ‘hot dog headache, and Alzheimer’s disease [76], Acute MSG(500 mg/kg i.p.) administration disrupted norepinephrine, dopamine, glycine , glutamate, asparate neurotransmitter contents led to apparent reduction in both the hypothalamus (16%) and cerebellum of adult rats, but was increased in aged rats (24 month old). Cortical serotonergic deficits were reported in aged rats. Aged rats possessed alterations in amino acids, especially the excitatory amino acids such as glutamate and aspartate [77]. Glutamate excitotoxicity is greatly associated with neurodegenerative disorders including amyotrophic lateral sclerosis, multiple sclerosis and Parkinson’s disease [78].
Dief et al. [58], reported that oral or subcutaneous administration of MSG increased b-amyloid accumulation in the rat hippocampus and FAS ligand and decreased adenosine monophosphate-activated protein kinase which is associated with increased anxiety. Soluble oligomers of the amyloid-β peptide (AβOs) was found to accumulate in brain of Alzheimer’s disease and promote extracellular accumulation of glutamate and d-serine, a co-agonist at glutamate receptors of the N-methyl-d-aspartate subtype (NMDARs), in hippocampal neuronal cultures [79]. Amyloid-β peptide was involved in generating reactive oxygen species (ROS), leading to inhibition of the glutamate transporter protein by the glutamatergic neurons [80].In patients with amyotrophic lateral sclerosis, Alzheimer’s and Huntington’s disease, there was a marked decrease of transmission of glutamate uptake in synaptosomes from spinal cord, motor cortex, and somatosensory cortex, and missing in visual cortex, striatum, or hippocampus [81].
Administration of monosodium glutamate to rat newborn led to dramatic cerebral and striatum neuronal cell death by either apoptosis or via MAPK p38 pathway activation in 8, 10, and 14 day-old. This drastic alterations was inhibited by pre-treatment with SB203580 (p38 inhibitor). These findings led the authors to explain that neuronal death induced by glutamate was promoted by p38 pathway activated by TNF-alpha [82].
Individual or combined monosodium glutamate (8mg/kg) and aspartame-treatment (32 mg/kg) for one month led to significant disruption of cognitive responses, memory retention and learning capabilities as well as decreased significantly the levels of neurotransmitters (dopamine and serotonin) and increased lipid peroxidation [83].
Investigating hippocampal slice cultures of rat, characterized by high branched axons and overexpression of glutamate transporters. Using confocal microscopy, the mobility of mitochondria within the astrocytic processes and neuronal dendrites was markedly greater compared to astrocytes. Inhibition of glutamate transport and Na(+)/Ca(2+) exchange activated the mobility of mitochondria in astrocytes [84].
Six-month-old mice treated with monosodium glutamate (MSG) developed a model of obesity-induced pre-diabetes, characterized by increased tau phosphorylation at Ser396 and Thr231 in the hippocampus which contributed to the formation of neurofibrillary tangles related to cognitive dysfunction and Alzheimer’s disease. Subcutaneous administration of a lipidized analog of prolactin-releasing peptide (palm-PrRP31) for two weeks increased phosphorylation of the insulin cascade kinases PDK1 (Ser241), Akt (Thr308), GSK-3β (Ser9) and attenuated phosphorylation at Ser396, Thr231, and Thr212 of tau kinases in the hippocampi [85].
Monosodium glutamate treatment increased the [3H] serotonin (5-HT) uptake in the cerebral cortices of rats and induced a deregulation of hypothalamic pituitary adrenal axis function (by increasing serum MSG treatment increased the [3H]5-HT uptake in the cerebral cortices of rats and induced a deregulation of HPA axis function (by increasing serum ACTH and corticosterone levels) [86].
The brain need high-energy requirements and blockage of blood flow causes rapid deterioration of brain cells. Acidosis and liberation of excess glutamate are of two excitotoxic mechanism for induction of brain ischemia. Overexpression of channelrhodopsin-2 in glial cells led to glial acidification and to release of glutamate and increase the severity of brain damage. However, glial alkalization via optogenetic activation of a proton pump, archaerhodopsin (ArchT), led to cessation of glutamate release and improve the disease [87].
High concentration of glutamate (Glu) is excitotoxic for nervous system structures. This may lead to glial reactivity ie. increased expression of glial fibrillary acidic protein (GFAP) and S100β protein, and also to hypertrophy and proliferation of cells which are determined by the presence of Ki-67 antigen. Its administration to young rats (2 g/kg b.w. and 4 g/kg b.w.) caused marked alterations of astrocytes with the GFAP expression in the SLM of the hippocampal CA1 region. Also, there is marked increase in the number of GFAP and S100β immunopositive astrocytes and nuclei with Ki-67 expression [88].
The cellular and optical densities of GFAP-immunoreactive sections of suprachiasmatic nucleus were significantly increased in neonatal rats received monosodium glutamate (3.5 mg/g/day,sc) for 3-10 days [89].
In vitro studies of MSG (20 mM) on astrocyte culture cells revealed increased liberation of reactive oxygen species and apoptotic cell death and attenuated by N-acetylcysteine (500 μM)-treatment restoration of mitochondrial membrane potential and intracellular reduced glutathione and up-regulation of endoplasmic reticulum stress markers [90].
Within the neurons, there is a greater percentage of mobility of mitochondria than in astrocytes and are present at sites of high metabolic activity. Glutamate transport and the concomitant activation of the Na(+)/K(+)-ATPase represent a substantial energetic demand on astrocytes. Similar to inhibitory effect of glutamate, inhibiting neuronal activity with tetrodotoxin (TTX) increased the percentage of mobile mitochondria in astrocytes [91].
Phytotherapy of glutamate neurotoxicity
Phytotherapy involve the wide application of medicine which is of great importance and less side than the organochemical compounds. Hydrogen peroxide and peroxynitrite impaired glutamate uptake by astrocytes dependent on the concentration level and increased following inhibition of catalase. This led to marked increase of neurotoxicity. Carob-supplementation before or followed monosodium glutamate treatment reduced the accumulation of hydrogen peroxide in the extracellular space of the assayed brain tissues, and exerted a potential therapeutic effects [92].
Administration of MSG to neonatal rats increased body mass index and serum glucose Špolcová et al. [85], mentioned abnormally hyperphosphorylated tau protein at Ser396 and Thr231 in the hippocampus in monosodium glutamate-obese mice post-6-month-treatment and improved post palm-PrRP31 and liraglutide-treatment.
Cinnamon (CE) (the phenolic component of carob fruit) supplementation improved Alzheimer’s disease (AD) previously induced by MSG-treatment for 10 months. It is characterized by insulin sensitivity, decreased phosphorylated glycogen synthase kinase-3β (pGSK3β), and increased the cholinesterase activity, cognition and hippocampus neuronal cell loss in non-transgenic rat model of AD rats [93].
Ferulic acid (carob polyphenol) [21], is a novel neuroprotector. It is protected against the MSG-induced hippocampal lesions characterized by intracellular edema, degeneration and necrosis of neurons, and hyperplasia [94]. It inhibited apoptotic morphology, active caspase-3 protein expression, and PARP cleavage induced by glutamate-treatment [95].
Many plant- extracts and their chemical constituents are reported to have beneficial effects on brain function [96], (Kennedy and Wightman, 2011). Carob-supplementation before or followed monosodium glutamate treatment reduced the accumulation of hydrogen peroxide in the extracellular space of the assayed brain tissues, and exerted a potential therapeutic effects [97].
Several phytochemical components such as ferulic acid [98], and epigallocatechin-3-gallate [99], protects against free radical mediated cell damage. Also, carob constituents ferulic acid improved AD through reduction of amyloid beta (Abeta) and AChE levels in the hippocampus related to development of cognition induced by glutamate [100].
Alzheimer and Parkinson are developed from increased oxidative stress. Ferulic acid is a natural antioxidant [101]. Its clinical importance in treatment Alzheimer’s disease resulted from maintaining cell viability, increased superoxide dismutase, and inhibited the production of tumor necrosis factor-α and interleukin -1β induced amyloid-beta peptide 25-35(Aβ25-30) formation [102].
One-year-old mice with established β-amyloid plaques received daily doses of OG and FA alone or in combination for 3 months. APP/PS1 mutant transgenic mice received dimeric derivatives of ferulic acid KMS4001 at doses of 3 and 30mg/kg/day via drinking water showed the significantly enhanced novel-object recognition memory at both 1.5 and 3 months and decreased amyloid peptide Aβ1-40 and Aβ1-42 levels in the frontal cortex [103].
PSAPP mice receiving combination therapy of octyl gallate (OG) and ferulic acid (FA) for 3 months had statistically significantly improved cognitive function through reductions of β-amyloid deposits in brain parenchymal and cerebral vascular tissues the main cause of Alzheimer’s disease [104]. A series of novel Novel Tacrine-Ferulic Acid Hybrids [105], and ferulic acid-O-alkylamines derivatives were proved to be a good choice against Alzheimer’s disease [106].
Cinnamon (CE) (the phenolic component of carob fruit) supplementation improved Alzheimer’s disease (AD) induced by MSG-treatment in non-transgenic rat model of AD rats [107].
Chloroform: methanolic (80:20) extract of C. asiatica (CA; 100 and 200 mg/kg),improved monosodiumglutamate impaired locomotor activity and CAl a region of the hippocampus coincides with impaired lipid peroxides and ameliorated catalase, super oxide desmutase and lipid peroxides levels in hippocampus and striatum regions [108]. MSG (2 g/kg, 7 days i.p.) -treatment for seven days decreased the activities of SOD and increased malondialdhyde in serum, brain, liver and kidney of Sprague-Dawley female rats and improved after tannic acid-treatment (50 mg/kg, 3 days) [109].
Catechol is known as pyrocatechol or 1,2-dihydroxybenzene, Its derivatives including 3-methylcatechol, 4-methylcatechol, and 4-tert-butylcatechol exerted downregulation of lipopolysaccharide (LPS)-induced NO and tumor necrosis factor (TNF)-alpha production in BV-2 microglia cells through inhibition of inducible nitric oxide synthase (iNOS) and TNF-alpha at mRNA or protein levels [110].
Protocatechuic acid was found to protect neurotoxicity against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) through the depletion of dopamine (DA) and its metabolites in striatum. It is ameliorated the histopathology in substantia nigra and the downexpression of tyrosine hydroxylase in f C57BL/6J mice [111].
Chlorogenic acid a polyphenol of carob fruit protect against glutamate-induced neuronal cell death in invitro cultures of mouse cerebral cortex through inhibition of release of intracellular concentrations of Ca(2+) and nitric oxide the causes of neuronal cell death [112].Several dtudies reported that the Chlorogenic acid exerted therapeutic potential including neuroprotection, cardioprotection, weight loss, chemopreventive properties, anti-inflammatory activity, decreased blood pressure, decreased diet-induced insulin resistance, decreased blood pressure, anxiolytic effects, and antihyperalgesic effects [113]. The neuroprotective properties of Chlorogenic acid is occurred by inhibiting acetylcholinesterase and butyrylcholinesterase, activities as well as preventing oxidative stress-induced neurodegeneration [114,115].
Glutamate and nitric oxide (NO) are active regulators of dendrite and axon development in the brain. Excess glutamatergic stimulation induced neuronal atrophy and shrinkage with eventual neurodegeneration and cell death. Twenty-four hours-treatment of cultured primary cortical rat neurons with glutamate (500μM) or N-methyl-d-aspartate (NMDA) (100-500μM) combined with glycine impair neurite outgrowth [116].
It is known that glutamate is the main excitatory neurotransmitter in the brain and over-activation of the glutamate receptors, NMDA, AMPA and kainate (KA), led to neuronal death in epilepsy, seizures and neurodegenerative diseases. Mitochondria is responsible for neuronal excitability, including managing Ca(2+) homeostasis and ATP production to maintain Na(+)K(+)-ATPase in the central nervous system. Also, it is the primary site of reactive oxygen species production enhancing cell damage and causing oxidative stress. Resveratrol was found to decrease intracellular ROS production, through NMDA, AMPA/KA, intracellular Ca(2+) and the heme oxygenase 1 (HO1) pathway [117].
The author finally concluded that carob micro-constituents showed phytotherapeutic potential against neurotoxicity.
- Amor S, Puentes F, Baker D, van der Valk P (2010) Inflammation in neurodegenerative diseases. Immunology 129: 154-169. Link: https://goo.gl/72KaPr
- Loliger J (2000) Function and importance of glutamate for savory foods. J Nutr 130: 915S-920S. Link: https://goo.gl/KjnKFa
- Finger TE (2009) International Symposium on Olfaction and Taste. Hoboken, NJ: The Annals of the New York Academy of Sciences. 1170. Link: https://goo.gl/3DW2bt
- Schiffman SS (1991) Taste and smell perception in elderly persons. In J.E. Fielding & H.I. Frier (eds.), Nutritional Needs of the Elderly. New York: Raven Press. 61-73.
- Schiffman SS (1983) Taste and smell in disease. N Engl J Med 308:1275-1279. Link: https://goo.gl/ruo3NG
- Schiffman SS (1996) Update on monosodium glutamate: Sensory properties and safety. Nutr 96: 451-452.
- Fowler CJ, Griffiths D, de Groat WC (2008) The neural control of micturition. Nat Rev Neurosci 9: 453–466. Link: https://goo.gl/ZQWqrG
- Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders . Nat Rev Neurosci 12: 723–738. Link: https://goo.gl/sVpf63
- Vazana U, Veksler R, Pell GS, Prager O, Fassler M, et al. (2016) Glutamate-mediated blood–brain barrier opening :implication for neuroprotection and drug delivery. J Neurosci 36: 7727–7739. Link: https://goo.gl/BHWVas
- Diniz YS, Faine LA, Galhardi CM, Rodrigues HG, Ebaid GX, et al. (2005) Monosodium glutamate in standard and high-fiber diets: metabolic syndrome and oxidative stress in rats. Nutrition 21: 749–755. Link: https://goo.gl/t5cQy5
- Rotimi OA, Olayiwola IO, Ademuyiwa O, Balogun EA (2012) Effects of fibre-enriched diets on tissue lipid profiles of MSG obese rats. Food Chem Toxicol 50: 4062–4067. Link: https://goo.gl/4iK9vp
- Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, et al (2006) Elevated monoamine oxidase A levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry 63: 1209–1216. Link: https://goo.gl/WJevap
- Ge JF, Qi CC, Zhou JN (2013) Imbalance of leptin pathway and hypothalamus synaptic plasticity markers are associated with stress-induced depression in rats. Behav Brain Res 249: 38–43. Link: https://goo.gl/VuDcft
- Sheldon AL, Robinson MB (2007) The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int 51: 333–355. Link: https://goo.gl/X17ruV
- Hazell AS (2009) Astrocytes are a major target in thiamine deficiency and Wernicke’s encephalopathy. Neurochem Int 55: 129–135. Link: https://goo.gl/d1qcf4
- Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, et al.(1999) Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol 43: S102-128. Link: https://goo.gl/HWyRPD
- Park E, Yu KH, Kim DK, Kim S, Sapkota K, et al. (2014) Protective effects of N-acetylcysteine against monosodium glutamate-induced astrocytic cell death. Food Chem Toxicol 67: 1-9. Link: https://goo.gl/ZTePqG
- El Batal H, Hasib A, Ouatmane A, Boulli A, Dehbi F, et al. (2013) Yield and composition of carob bean gum produced from different Moroccan populations of carob ( Ceratonia siliqua L.). J Mater Environ Sci 4: 309-314. Link: https://goo.gl/wU4uAn
- Papagiannopoulos M, Wollseifen HR, Mellenthin A, Haber B , Galensa R (2004) Identification and quantification of polyphenols in Carob Fruits ( Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MS n . J Agric Food Chem 52: 3784–3791. Link: https://goo.gl/tuNiSM
- Youssef MKE, El-Manfaloty MM, Ali HM (2013) Assessment of Proximate Chemical Composition, Nutritional Status, Fatty Acid Composition and Phenolic Compounds of Carob ( Ceratonia siliqua L.). Food and Public Health 3: 304-308. Link: https://goo.gl/SdNX1r
- Bravo L, Grados N, Saura - Calixo F (1998) Characterization of syrups and dietary fiber obtained from Mesquite pods (Prosopis pallida L). J Agric Food Chem 46: 1727 – 1733. Link: https://goo.gl/GEuQEw
- Makris DP, Kefalas P (2004) Carob Pods ( Ceratonia siliqua L.) as a Source of Polyphenolic Antioxidants. Food Technol Biotechnol 42 105–108. Link: https://goo.gl/AaJKWG
- Gruendel S, Otto B, Garcia AL, Wagner K, Mueller C, et al. (2007) Carob pulp preparation rich in insoluble dietary fibre and polyphenols increases plasma glucose and serum insulin responses in combination with a glucose load in humans. Br J Nutr 98: 101–105. Link: https://goo.gl/Kch3wn
- Klenow S, Glei M, Haber B, Owen R, Pool-Zobel BL (2008) Carob fiber compounds modulate parameters of cell growth differently in human ht29 colon adenocarcinoma cells than in LT97 colon adenoma cells. Food Chem Toxicol 46: 1389–1397. Link: https://goo.gl/RUQXWJ
- Sigge GO, Lipumbua L, Britza TJ (2011) Proximate composition of carob cultivars growing in south africa. S Afr J Plant Soil 28: 17–22. Link: https://goo.gl/P2VbVx
- Dakia PA, Wathelet B, Paquot M (2007) Isolation and chemical evaluation of carob ( Ceratonia siliqua L.) seed germ. Food Chem 102: 1368–1374. Link: https://goo.gl/VUWM2o
- Cappa C, Lucisano M, Mariotti M (2013) Influence of Psyllium, sugar beet fibre and water on gluten-free dough properties and bread quality. Carbohydr Polym 98: 1657-1666. Link: https://goo.gl/5bemmB
- Avallone R, Cosenza F, Farina F, Baraldi C, Baraldi M (2002) Extraction and purification from Ceratonia siliqua of compounds acting on central and peripheral benzodiazepine receptors. Fitoterapia 73: 390-396. Link: https://goo.gl/P5Lqmp
- Sebai H, Souli A, Chehimi L, Rtibi K, Amri M, et al.(2013) In vitro and in vivo antioxidant properties of Tunisian carob ( Ceratonia siliqua L.). J Med Plants Res 7: 85-90. Link: https://goo.gl/KXyGwN
- Bengoechea C, Romero A, Villanueva A, Moreno G, Alaiz M, et al. (2008) Composition and structure of carob ( Ceratonia siliqua L.) germ proteins. Food Chem 107: 675–683. Link: https://goo.gl/PKLpxM
- Kumazawa S, Taniguchi M, Suzuki Y, Shimura M, Kwon MS, et al.(2002) Antioxidant activity of polyphenols in carob pods. J Agric Food Chem 50: 373-377. Link: https://goo.gl/UVfy3f
- Ayaz FA, Torun H, Glew RH, Bak ZD, Chuang LT, et al. (2009) Nutrient content of carob pod ( Ceratonia siliqua L.) flour prepared commercially and domestically. Plant Foods Hum Nutr 64: 286-292. Link: https://goo.gl/AJENVe
- El Hajaji H, Lachkar N , Alaoui K , Cherrah Y , Farah A , et al. (2010) Antioxidant Properties and Total Phenolic Content of Three Varieties of Carob Tree Leaves from Morocco. Rec Nat Prod 4: 193-204. Link: https://goo.gl/VMd7M8
- Souli A, Sebai H, Chehimi L, Rtibi K, Tounsi H, et al. (2015) Hepatoprotective effect of carob against acute ethanol-induced oxidative stress in rat. Toxicol Ind Health 31: 802-810. Link: https://goo.gl/r2G2GT
- Sassi A, Bouhlel I, Mustapha N, Mokdad-Bzeouich I, Chaabane F, et al. (2016) Assessment in vitro of the genotoxicity, antigenotoxicity and antioxidant of Ceratonia siliqua L. extracts in murine leukaemia cells L1210 by comet assay. Regul Toxicol Pharmacol 77: 117-124. Link: https://goo.gl/VxDBFK
- Beyreuther K, Biesalski HK, Fernstrom JD, Grimm P, Hammes WP, et al. (2007) Consensus meeting: monosodium glutamate – an update. Eur J Clin Nutr 61: 304–313. Link: https://goo.gl/5dhncR
- Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, et al (2006) Elevated monoamine oxidase A levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry 63: 1209–1216. Link: https://goo.gl/ZJKug6
- Olney JW ( 1989 ) Glutamate, a neurotoxic transmitter. J Child Neurol 4: 218–226. Link: https://goo.gl/6JoeSu
- Boeck CR, Kroth EH, Bronzatto MJ, Vendite D (2005) Adenosine receptors co-operate with NMDA preconditioning to protect cerebellar granule cells against glutamate neurotoxicity. Neuropharmacology 49: 17-24. Link: https://goo.gl/i2quRQ
- Yawata I, Takeuchi H, Doi Y, Liang J, Mizuno T, et al. (2008) Macrophage induced neurotoxicity is mediated by glutamate and attenuated by glutaminaseinhibitors and gap junction inhibitors. Life Sci 82: 1111-1116. Link: https://goo.gl/xdBZiW
- Schwendt M, Jezova D (2001) Glutamate receptors and transporters in the brain and peripheral tissues. Cesk Fysiol 50: 43–56. Link: https://goo.gl/qRwCWu
- Sun Y, Olson R, Horning M, Armstrong N, Mayer M, et al. (2002) Mechanism of glutamate receptor desensitization. Nature 417: 245-253. Link: https://goo.gl/zoQb6j
- Hinoi E, Takarada T, Ueshima T, Tsuchihashi Y, Yoneda Y (2004) Glutamate signaling in peripheral tissues. Eur J Biochem 271: 1–13. Link: https://goo.gl/p2b5E3
- Cho DW (1985) Glutamate neurotoxicity in cortical cell culture is calcium dependent, Neurosct Lett 58: 293-297 . Link: https://goo.gl/eUvLSx
- Meyerson JR, Kumar J, Chittori S, Rao P, Pierson J, et al. (2014) Structural mechanism of glutamate receptor activation and desensitization. Nature 514: 328-334. Link: https://goo.gl/DtbJwB
- Petroff OA (2002) GABA and glutamate in the human brain. Neuroscientist 8: 562–573. Link: https://goo.gl/HPf77B
- Collingridge GL, Olsen RW, Peters J, Spedding M (2009) A nomenclature for ligand-gated ion channels. Neuropharmacology 56: 2–5. Link: https://goo.gl/WRww4r
- Julio-Pieper M, Flor PJ, Dinan TG , Cryan JF (2011) Exciting times beyond the brain: Metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol Rev 63: 35-58. Link: https://goo.gl/ArSh5u
- Greger IH, Ziff EB, Penn AC (2007) Molecular determinants of AMPA receptor subunit assembly. Trends Neurosci 30: 407–416. Link: https://goo.gl/wfBwaf
- Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51: 7–61. Link: https://goo.gl/cmpVne
- Li F, Tsien JZ (2009) Memory and the NMDA receptors. N Engl J Med 361: 302–303. Link: https://goo.gl/wpEiKZ
- Hansen KB, Naur P, Kurtkaya NL, Kristensen AS, Gajhede M, et al. (2009) Modulation of the dimer interface at ionotropic glutamate-like receptor delta2 by D-serine and extracellular calcium. J Neurosci 29: 907–917. Link: https://goo.gl/SUygCA
- Yuzaki M (2013) Cerebellar LTD vs. motor learning-lessons learned from studying GluD2. Neural Networks 47: 36–41. Link: https://goo.gl/H7Zbwv
- Vaccaro M, Riva C, Tremolizzo L, Longoni M, Aliprandi A, et al. (2007) Platelet glutamate uptake and release in migraine with and without aura. Cephalalgia 27: 35– 40. Link: https://goo.gl/Z7VYHv
- Hawkins RA (2009) The blood-brain barrier and glutamate. Am J Clin Nutr 90: 867S–874S. Link: https://goo.gl/Hf4Vhi
- Shigeri Y, Seal RP, Shimamoto K (2004) Molecular pharmacology of glutamate transporters EAATs and VGLUTs. Brain Res Rev 45: 250–265. Link: https://goo.gl/Ev7f7N
- Graham TE, Sgro V, Friars D, Gibala MJ (2000) Glutamate ingestion: the plasma and muscle free amino acid pools of resting humans. Am J Physiol Endocrinol Metab 278: E83–E89. Link: https://goo.gl/532QRE
- Shindo Y, Fujimoto A, Hotta K, Suzuki K, Oka K (2010) Glutamate-induced calcium increase mediates magnesium release from mitochondria in rat hippocampal neurons. J Neurosci Res 88: 3125-3232. Link: https://goo.gl/FsFJX8
- Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105. Link: https://goo.gl/CceTjr
- Yenari MA, Han HS (2012) Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 13: 267-278. Link: https://goo.gl/txMwgP
- Meldrum BS (1994) The role of glutamate in epilepsy and other CNS disorders. Neurology 44: S14-S23. Link: https://goo.gl/ZHnMkk
- Lu H, Liu X, Deng Y, Qing H (2013) DNA methylation, a hand behind neurodegenerative diseases. Front Aging Neurosci 5: 85. Link: https://goo.gl/Hd8Cae
- Greenberger LM, Besharse JC (1985) Stimulation of photoreceptor disc shedding and pigment epithelial phagocytosis by glutamate, aspartate, and other amino acids. J Comp Neurol 239: 361-372. Link: https://goo.gl/ZNz9PP
- Chambille I, Serviere J (1993) Neurotoxic effects of neonatal injections of monosodium L-glutamate (L-MSG) on the retinal ganglion cell layer of the golden hamster: anatomical and functional consequences on the circadian system. J Comp Neurol 338: 67-82. Link: https://goo.gl/t4UVaj
- Wakabayashi Y, Yagihashi T, Kezuka J, Muramatsu D, Usui M, et al. (2006) Glutamate levels in aqueous humor of patients with retinal artery occlusion. Retina 26: 432-436. Link: https://goo.gl/2Rq2GP
- Diederen RM, La Heij EC, Deutz NE, Kijlstra A, Kessels AG, et al. (2006) Increased glutamate levels in the vitreous of patients with retinal detachment. Exp Eye Res 83: 45-50. Link: https://goo.gl/XKhJPR
- Kiss P, Atlasz T, Szabadfi K, Horvath G, Griecs M, et al. (2011) Comparison between PACAP- and enriched environment-induced retinal protection in MSG-treated newborn rats. Neurosci Lett 487: 400-405. Link: https://goo.gl/MBdmBm
- Wei L, Ge L, Qin S, Shi Y, Du C, et al. (2012) Hydrogen-rich saline protects retina against glutamate-induced excitotoxic injury in guinea pig. Exp Eye Res 94: 117-127. Link: https://goo.gl/KjkRtC
- Dief AE, Kamha ES, Baraka AM, Elshorbagy AK (2014) Monosodium glutamate neurotoxicity increases beta amyloid in the rat hippocampus: a potential role for cyclic AMP protein kinase. Neurotoxicology 42: 76-82. Link: https://goo.gl/6GkwYD
- Xu L, Zhao Y, Zhan SQ, Wang HS, Shi WC (2002) Expression of bax and bcl-2 in mouse offspring brain after maternal oral administration of monosodium glutamate. J Xi’an Med Univ 14: 38–42. Link: https://goo.gl/t95Kou
- Tóth L, Karcsu S, Feledi J, Kreutzberg GW (1987) Neurotoxicity of monosodium-l-glutamate in pregnant and fetal rats. Acta Neuropathologica 75: 16-22. Link: https://goo.gl/L2adh3
- Bawari M, Babu GN, Ali MM, Misra UK (1995) Effect of neonatal monosodium glutamate on lipid peroxidation in adult rat brain. Neuroreport 6: 650-652. Link: https://goo.gl/NUJX6q
- Manivasagam T , Subramanian P (2004) Influence of monosodium glutamate on circadian rhythms of lipid peroxidation products and antioxidants in rats. Ital J Biochem 53: 23-27. Link: https://goo.gl/et2mKt
- Goldsmith PC (2000) Neuroglial responses to elevated glutamate in the medial basal hypothalamus of the infant mouse. J Nutr 130: 1032S-1038S . Link: https://goo.gl/fRV2je
- Chamoun R, Suki D, Gopinath SP, Goodman JC, Robertson C (2010) Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J Neurosurg 113: 564-570. Link: https://goo.gl/Wz5MTG
- Cowburn R, Hardy J, Roberts P, Briggs R (1988) Presynaptic and postsynaptic glutamatergic function in Alzheimer's disease. Neurosci Lett 86: 109-113. Link: https://goo.gl/LsQfXv
- Scher W, Scher BM (1992) A possible role for nitric oxide in glutamate (MSG)-induced Chinese restaurant syndrome, glutamate-induced asthma, 'hot-dog headache', pugilistic Alzheimer's disease, and other disorders. Med Hypotheses 38: 185-188. Link: https://goo.gl/yhPZqK
- Wallace DR, Dawson R (1990) Effect of age and monosodium-L-glutamate (MSG) treatment on neurotransmitter content in brain regions from male Fischer-344 rats. Neurochem Res 15: 889-898. Link: https://goo.gl/JoordA
- Lau A, Tymianski M (2010) Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch 460: 525-542. Link: https://goo.gl/BGcnvE
- Brito-Moreira J, Paula-Lima AC, Bomfim TR, Oliveira FB, Sepúlveda FJ, et al. (2011) Aβ oligomers induce glutamate release from hippocampal neurons. Curr Alzheimer Res 8: 552-562. Link: https://goo.gl/Kkvw5F
- Lauderback CM, Harris-White ME, Wang Y, Pedigo NW, Carney JM, et al. (1999) Amyloid beta-peptide inhibits Na+-dependent glutamate uptake. Life Sci 65: 1977–1981. Link: https://goo.gl/4Y7Ddu
- Rothstein JD, Martin LJ, Kuncl RW (1992) Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med 326: 1464-1468. Link: https://goo.gl/g1CStx
- Segura Torres JE, Chaparro-Huerta V, Rivera Cervantres MC, Montes-González R, Flores Soto ME, et al. (2006) Neuronal cell death due to glutamate excitotocity is mediated by p38 activation in the rat cerebral cortex. Neurosci Lett 403: 233-238. Link: https://goo.gl/V4yXpL
- Abu-Taweel GM, A ZM, Ajarem JS, Ahmad M (2014) Cognitive and biochemical effects of monosodium glutamate and aspartame, administered individually and in combination in male albino mice. Neurotoxicol Teratol 42: 60-67. Link: https://goo.gl/6Dw7vw
- Jackson JG, O'Donnell JC, Takano H, Coulter DA, Robinson MB (2014) Neuronal activity and glutamate uptake decrease mitochondrial mobility in astrocytes and position mitochondria near glutamate transporters. J Neurosci 34: 1613-1624. Link: https://goo.gl/5nTJ73
- Špolcová A, Mikulášková B, Holubová M, Nagelová V, Pirnik Z, et al. (2015) Anorexigenic lipopeptides ameliorate central insulin signaling and attenuate tau phosphorylation in hippocampi of mice with monosodium glutamate-induced obesity. J Alzheimers Dis 45: 823-835. Link: https://goo.gl/oY9HR3
- Quines CB, Rosa SG, Da Rocha JT, Gai BM, Bortolatto CF, et al. (2014) Monosodium glutamate, a food additive, induces depressive- like and anxiogenic-like behaviors in young rats. Life Sci 107: 27-31. Link: https://goo.gl/1kihyU
- Beppu K, Sasaki T, Tanaka KF, Yamanaka A, Fukazawa Y, et al. (2014) Optogenetic countering of glial acidosis suppresses glial glutamate release and ischemic brain damage. Neuron 81: 314-320. Link: https://goo.gl/kG1Ucs
- Krawczyk A, Jaworska-Adamu J, Rycerz K (2015) Immunohistochemical evaluation of hippocampal CA1 region astrocytes in 10-day-old rats after monosodium glutamate treatment. Pol J Vet Sci 18: 767-774. Link: https://goo.gl/yyjfjo
- Rojas-Castañeda JC, Vigueras-Villaseñor RM, Chávez-Saldaña M, Rojas P, Gutiérrez-Pérez O, et al. (2016) Neonatal exposure to monosodium glutamate induces morphological alterations in suprachiasmatic nucleus of adult rat. Int J Exp Pathol 97: 18-26. Link: https://goo.gl/XHe9VT
- Chen CJ, Liao SL, Kuo JS (2000) Gliotoxic action of glutamate on cultured astrocytes. J Neurochem 75: 1557-1565. Link: https://goo.gl/VgzdL4
- Jackson JG, O’Donnell JC, Takano H, Coulter DA, Robinson MB (2014) Neuronal Activity and Glutamate Uptake Decrease Mitochondrial Mobility in Astrocytes and Position Mitochondria Near Glutamate Transporters. J Neurosci 34: 1613–1624. Link: https://goo.gl/Axy76w
- Song I, Huganir RL (2002) Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 25: 578–88. Link: https://goo.gl/9ibxpL
- Madhavadas S, Subramanian S (2016) Cognition enhancing effect of the aqueous extract of Cinnamomum zeylanicum on non-transgenic Alzheimer's disease rat model: Biochemical, histological, and behavioural studies. Nutr Neurosci 16: 1-12. Link: https://goo.gl/nfhaC2
- Yu L, Zhang Y, Ma R, Bao L, Fang J, Yu T (2006) Potent protection of ferulic acid against excitotoxic effects of maternal intragastric administration of monosodium glutamate at a late stage of pregnancy on developing fetal brain. Eur Neuropsychopharmacol 16: 170-177. Link: https://goo.gl/69VrxG
- Jin Y, Yan EZ, Fan Y, Guo XL, Zhao YJ, et al. (2007) Neuroprotection by sodium ferulate against glutamate induced apoptosis is mediated by ERK and P13 kinase pathways. Acta Pharmacol Sin 28: 1881-1890. Link: https://goo.gl/eLyMYF
- Kennedy DO, Wightman EL (2011) Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv Nutr 2: 32–50. Link: https://goo.gl/XWA8cL
- Song I, Huganir RL (2002) Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 25: 578–88. Link: https://goo.gl/rctqv1
- Kanaski J, Aksenova M, Stoyanova A, Butterfield D A (2002) Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro : structure activity studies. J Nutr Biochem 13: 273–281. Link: https://goo.gl/f2dYzY
- Tosetti F D, Noonan M, Albini A (2009) Metabolic regulation and redox activity as mechanisms for angioprevention by dietary phytochemicals. Int J Cancer 125: 1997–2003. Link: https://goo.gl/YjakjX
- Mori T, Koyama N, Guillot-Sestier MV, Tan J, Town T (2013) Ferulic acid is a nutraceutical β-secretase modulator that improves behavioral impairment and alzheimer-like pathology in transgenic mice. PLoS ONE 8: e55774. Link: https://goo.gl/BZQz5T
- Nabavi SF, Devi KP, Malar DS, Sureda A, Daglia M, Nabavi SM (2015) Ferulic acid and Alzheimer's disease: promises and pitfalls. Mini Rev Med Chem 15(9):776-88. Link: https://goo.gl/33AFGj
- Huang H, Ma ZC, Wang YG, Hong Q, Tan HL, et al. (2015) Ferulic acid alleviates Aβ25-35- and lipopolysaccharide-induced PC12 cellular damage: a potential role in Alzheimer's disease by PDE inhibition. Int J Clin Pharmacol Ther 53: 828-837. Link: https://goo.gl/fsLk56
- Jung JS, Yan JJ, Li HM, Sultan MT, Yu J, et al. (2016) Protective effects of a dimeric derivative of ferulic acid in animal models of Alzheimer's disease. Eur J Pharmacol 782: 30-34. Link: https://goo.gl/uGXk3g
- Mori T, Koyama N, Tan J, Segawa T, Maeda M, et al. (2017) Combination Therapy with Octyl Gallate and Ferulic Acid Improves Cognition and Neurodegeneration in a Transgenic Mouse Model of Alzheimer Disease. J Biol Chem. Link: https://goo.gl/U61ved
- Fu Y, Mu Y, Lei H, Wang, Li X, et al. (2016) Design, Synthesis and Evaluation of Novel Tacrine-Ferulic Acid Hybrids as Multifunctional Drug Candidates against Alzheimer's Disease. Molecules 21 pii: E1338. Link: https://goo.gl/4bKEAE
- Sang Z, Pan W, Wang K, Ma Q, Yu L, et al. (2017) Design, synthesis and evaluation of novel ferulic acid-O-alkylamine derivatives as potential multifunctional agents for the treatment of Alzheimer's disease. Eur J Med Chem 130: 379-392. Link: https://goo.gl/82Q8o4
- Madhavadas S, Subramanian S (2016) Cognition enhancing effect of the aqueous extract of Cinnamomum zeylanicum on non-transgenic Alzheimer's disease rat model: Biochemical, histological, and behavioural studies. Nutr Neurosci. 2016: 1-12. Link: https://goo.gl/CFNnj6
- Ramanathan M, Sivakumar S, Anandvijayakumar PR, Saravanababu C, Pandian PR (2007) Neuroprotective evaluation of standardized extract of Centella asciatica in monosodium glutamate treated rats. Indian J Exp Biol 45: 425-431. Link: https://goo.gl/J2StBe
- Calis I U , Turgut Cosan D, Saydam F, Kerem Kolac U, et al. (2016) The effects of monosodium glutamate and tannic acid on adult rats. Iran Red Crescent Med J 18: e37912. Link: https://goo.gl/fEXfPv
- Zheng LT, Ryu GM, Kwon BM, Lee WH, Suk K (2008) Anti-inflammatory effects of catechols in lipopolysaccharide-stimulated microglia cells: inhibition of microglial neurotoxicity. Eur J Pharmacol 588: 106-113. Link: https://goo.gl/x2GtBT
- Zhang HN, An CN, Zhang HN, Pu XP (2010) Protocatechuic acid inhibits neurotoxicity induced by MPTP in vivo. Neurosci Lett 474: 99-103. Link: https://goo.gl/SYZno4
- Mikami Y, Yamazawa T (2015) Chlorogenic acid, a polyphenol in coffee, protects neurons against glutamate neurotoxicity. Life Sci 139: 69-74. Link:
- Oboh G, Agunloye OM, Akinyemi AJ, Ademiluyi AO, Adefegha SA (2013) Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer's disease and some pro-oxidant induced oxidative stress in rats' brain-in vitro. Neurochem Res 38: 413-419. Link: https://goo.gl/gLSLba
- Heitman E, Ingram DK (2017) Cognitive and neuroprotective effects of chlorogenic acid. Nutr Neurosci 20: 32-39. Link: https://goo.gl/ni3dhW
- Doucet MV, O'Toole E, Connor T, Harkin A (2015) Small-molecule inhibitors at the PSD-95/nNOS interface protect against glutamate-induced neuronal atrophy in primary cortical neurons. Neuroscience 301: 421-438. Link: https://goo.gl/ucmGx2
- Quincozes-Santos A, Bobermin LD, Tramontina AC, Wartchow KM, Tagliari B, et al. (2014) Oxidative stress mediated by NMDA, AMPA/KA channels in acute hippocampal slices: neuroprotective effect of resveratrol. Toxicol In Vitro 28: 544-551. Link: https://goo.gl/SrQF1x
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
