Global Journal of Zoology
Prenatal Stress Reduces Learning and Memory in Pre-pubertal, Young, and Adult Rats of Both Sexes
Guerrero Aguilera María de los Angeles1, Rubio Osornio María del Carmen2, Galván Arzate Sonia3 and Retana-Márquez Socorro4*
2Departamento de Neurofisiología, INNN “Manuel Velasco Suárez”, México
3Departamento de Neuroquímica, INNN “Manuel Velasco Suárez”, México
4Departamento de Biología de la Reproducción, Universidad Autónoma Metropolitana Iztapalapa, México
Cite this as
de los Angeles GAM, del Carmen ROM, Sonia GA, Socorro RM (2017) Prenatal Stress Reduces Learning and Memory in Pre-pubertal, Young, and Adult Rats of Both Sexes. Glob J Zool 2(1): 013-020 DOI: 10.17352/gjz.000006Prenatal stress (PS) induced by immobilization produces deficiencies in spatial learning and information retrieval. These deficiencies seem to be larger in males than in females, and have been explained as an effect of fetal exposure to high concentrations of maternal corticosterone during stress response. However, the effects of PS have only been assessed at a single time point and/or sex. In this work, the effect of PS on spatial learning and memory induced by immersion in cold water was evaluated in young and adult rats of both sexes. PS was induced during gestational days 15 through 21. Corticosterone in dams, body weight, corticosterone, learning and memory were assessed in male and female offspring at one, two, and three months of postnatal life. Results showed that escape latencies of PS rats of both sexes were longer as compared to those of control groups and that the number of platform-site crossovers and time spent in the platform quadrant were lower in the PS animals as compared to the control groups. Corticosterone levels were higher in PS females and males compared with controls. The body weight was decreased only in PS males of one month of age. These results show that PS by immersion in cold water alters learning and memory processes in the offspring, regardless of sex or age, since the effects are similar in females and males during youth and adulthood. These behavioral effects are related to high serum corticosterone.
Introduction
Prenatal stress (PS) is the result of fetal exposure to the elements of stress response in dams, and has been related to cognitive deterioration in the offspring during adulthood [1-3]. In humans, high glucocorticoid concentrations during the fetal stage are associated with the presence of a number of psychiatric pathologies in children and teenagers, such as schizophrenia [4], autism [5,6] attention deficit hyperactivity disorder [7-9], and learning and language deterioration [10-12]. Few of these studies account for gender differences and most observations have only been performed in male individuals [11,13]. Thus, experimental models in animals were developed to study the adverse effects of PS on cognitive functions [14]. PS deteriorates spatial learning and memory in male [15-17], but not in female rats [18]; in addition, PS eliminates the natural differences between sexes in cognitive function [19]. In three-month-old animals PS by immobilization [20], and two-month-old animals PS by a variety of short-term stressors (immobilization, forced swimming, and permanence on an elevated platform) [18], during the last gestational week, altered learning in male rats, but not in female, was reported. Another study showed that maternal physical stress deteriorates spatial learning and memory in rats of both sexes at 40 days of postnatal life, but maternal psychological stress only affects males [21]. Different results among studies may be explained in terms of the diverse protocols for PS used (varying in duration, intensity or gestational period) and/or the age of evaluation. Currently, there are no reports evaluating the effects of PS on learning and memory in animals of both sexes at different stages of postnatal life (prepubertal, young, and adult). Therefore, the objective of this work was to study whether PS applied during the last third of gestation alters cognitive performance in offspring of both sexes at one, two, and three months of age. Corticosterone was also evaluated in dams and offspring. We have evaluated corticosterone levels in dams exposed to stress in cold water and demonstrated that this paradigm is stressful for them [22].
Materials and Methods
Animals
Three-month-old Wistar pregnant female rats (n = 18) obtained from the vivarium of the Universidad Autónoma Metropolitana Iztapalapa were utilized. The animals were maintained in individual transparent Plexiglas cages at constant room temperature (22 ± 2 °C) with an inverted light/dark cycle (12 h /12 h; lights off at 1000) and free access to food and water.
Ethical declaration
Animal management and experiments were carried out in agreement with the Mexican norm (NOM-062-ZOO-1999), as well as the domestic and laboratory animal regulation established in the Guidelines for ethical conduct in research, teaching and dissemination of the Division of Biological Sciences and Health, published on May 2010.
Prenatal stress
During the last gestational week (from day 15 to 21), gestating dams assigned to the PS group (n = 10) went through two sessions of stress induced by immersion in cold water (ICW) at 10:00 and 15:00 h. Stress was induced by placing the rats individually in a Plexiglas cage filled with water at 15 °C and a height of 15 cm during 15 minutes. The cage was covered by a grid to prevent escape [23]. This stress procedure involves the combination of two well-known physical stressors: cold (15°C) and immersion in water. Additionally, it has a psychological component of no scape possibility during the exposure. Thus, the additive components of these stressful situations, and the duration of the exposure to this stressor are enough to cause an intense response of adrenal axis, even more intense than immobilization does, as has been reported earlier [24]. Dams assigned to the control group (CON, n = 8) were kept in their cages and only received routine cleaning. After delivery, litter sizes were evened out in every group. Offspring remained with dams until weaning from day 21 of postnatal life. The number of individuals per group was: for females, CON = 15 and PS = 30; and for males, CON = 15 and PS = 30.
Spatial navigation task
Learning and memory spatial performance of rats was measured using the Morris Water Maze (MWM) task. The MWM consisted of a circular pool, diameter of 170 cm and depth of 50 cm, surrounded by white walls. The pool was filled with water to a height of 30 cm and kept at a temperature of 21 ± 2 °C. The pool was divided into four equally-sized imaginary quadrants which were labeled according to the four cardinal points (NE for northeast, NW for northwest, SE for southeast, and SW for southwest) using the maze access as the reference. Three colorful pictures were set on the walls to serve as spatial clues. A transparent acrylic platform (19 cm X 22 cm) was placed 2 cm below the water level in the NE quadrant, and equidistantly to the wall and center of the pool.
At the three different ages, one (n=10), two (n=10), and three (n=10) months old, the animals performed daily learning sessions for four consecutive days (days 1 through 4) consisting of four trials per session. Forty eight hours after the learning sessions, animals performed a single-trial session (day 6); and 24 hours later (day 7), a memory trial without the platform, allowing the rats to swim for 60 seconds, was performed. All the sessions were video recorded. Thirteen days after the beginning of learning sessions (day 13), all the animals performed a test in the maze with the platform inside. Each trial started placing the rat on the surface of water with the snout pointing to the pool wall, in a quadrant different from the starting quadrant of previous trials. If the rat did not find the platform after 60 seconds, the rat was directed by the experimenter to the platform and left on the platform for 30 seconds [25]; this procedure was only performed in the first trial. When the rat found the platform within the 60-second limit, the rat was left on it for 30 seconds.
The behavioral parameters analyzed were: escape latency, defined as the time spent by the rat to reach the hidden platform; and, during the trials without a platform in the pool, time spent in the platform quadrant, the time spent by the rat swimming in the NE quadrant, and platform-site crossovers, being the number of times the rat swam over the specific former location of the platform.
Body weight registration
Body weight registration was daily performed during the first three months of postnatal life. Each animal was placed on a scale to determine its weight. Once the MWM trials started, the weight registration was performed before the learning and memory sessions.
Corticosterone concentrations
Blood samples were extracted from control (n=6) and stressed (n=6) dams, at gestational day 18. Blood samples were also obtained from progeny at 1, 2 and 3 months of age, after the last test at day 13. Animals were anesthetized with pentobarbital sodium (100 mg/kg; Pisabental, México), samples were extracted by cardiac puncture into test tubes and centrifuged at 3000 X g during 25 min at room temperature. Serum was frozen at -20 °C for corticosterone determination by HPLC. Corticosterone was extracted (Woodward and Emery 1987) from one mL of serum and mixed with 100 µl of the internal standard 19-nortestosterone (5 µg/mL in methanol/water, 60:40 v/v). The steroid was extracted into 5 mL of diethyl ether-dicloromethane (60:40, v/v) by vortex and centrifuged for 5 min. The organic phase was vortex mixed with 1 mL of HPLC-grade water and centrifuged again. The final organic phase obtained was evaporated under nitrogen at room temperature and then re-dissolved in 100 µl of methanol-water (60:40 v/v).
The chromatographic system consisted of a guard column (Symmetry C18, particle size 3.5 µm, 2.1 x 10 mm) and a column (Symmetry C18, particle size 5 µm, 2.0 x 150 mm, Waters Corp., Milford, Massachusetts). The system was equilibrated using HPLC-grade acetonitrile-water as a mobile phase (67:33 v/v) at a flow rate of 0.4 ml/min, and the separations were made at 40°C. Mobile phase was flushed by a system controller (Waters 600-MS), and corticosterone was assessed with 486 Waters UV absorbance detector set at 245 nm. A Millennium32 Workstation (Waters, Milford, Massachusetts) was used to analyze the results.
Chromatographic system calibration was performed using standards covering the range of 0-500 ng/dl for corticosterone in daily work. The regression lines between the peak heights and the concentrations of corticosterone were used for determining the unknowns. A typical regression line for corticosterone was: y = -0.6464+ 0.01655x (r = 0.99986).
Inter-assay and intra-assay coefficients of variation (C.V.) were determined using four plasma pools in the 15-500 ng/dl range for corticosterone, covering the normal range in rat plasma. The intra-day C.V. ranged from 1.5 to 2%, and the inter-day C.V. inter-day was 1.4 to 3.16%.
The detection limit of the assay for corticosterone was 5 ng/mL, using one ml of sample, at a signal to noise ratio of 2:1. The limit of quantitation for corticosterone was 10 ng/mL, and was determined with the formula: LOQ=10σ/s, where s is the slope of calibration curve and σ the S.D. of blank response.
Statistical analysis
All the statistical analyses were performed using the SPSS statistical software. MWM differences across trials were analyzed by means of an analysis of variance (ANOVA) for repeated measures. The analyses for time spent in the platform quadrant, platform-site crossovers, and body weight were performed using one-way ANOVA. The latency means in the MWM were analyzed utilizing a t-Student test and a one-way ANOVA, followed by the Newman-Keuls post-hoc test. The analysis of the percentage of animals that failed to found the platform site was performed using a Chi-squared test. Data were considered significant when p < 0.05.
Results
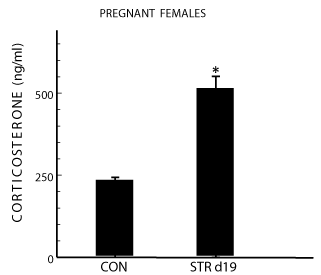
Serum corticosterone concentrations in pregnant females
Serum levels of Corticosterone in stressed pregnant rats were significantly higher than those in control pregnant females (t = -5.127, p < 0.0001) at day 19 of pregnancy (Figure 1).
Effects of prenatal stress on spatial learning and memory
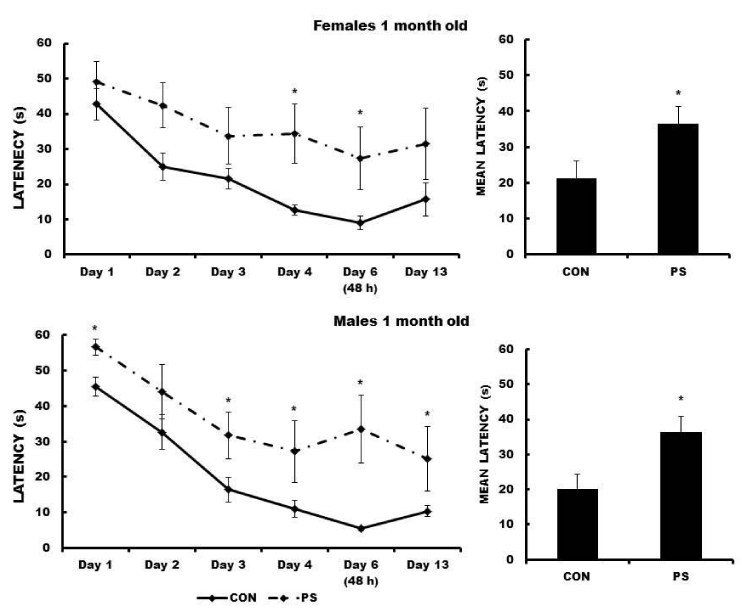
One-month-old PS females showed escape latencies significantly longer in days 4 and 6 of MWM training as compared to latencies of the corresponding female CON (F (5, 44) = 4.82, p < 0.048622). The mean escape latency of PS females was significantly longer as compared to the mean escape latency of the respective CON females (t = -3.8703, p < 0.000217; Figure 1). The escape latencies of the one-month-old PS males on days 1, 3, 4, and 13 were significantly longer than latencies of the corresponding CON males (F (5,44) = 6.89, p < 0.021003). PS animals swam around the maze most of the time. The mean escape latency of PS males was significantly longer than the mean of the respective CON males (t = -3.9713, p < 0.000146) (Figure 2).
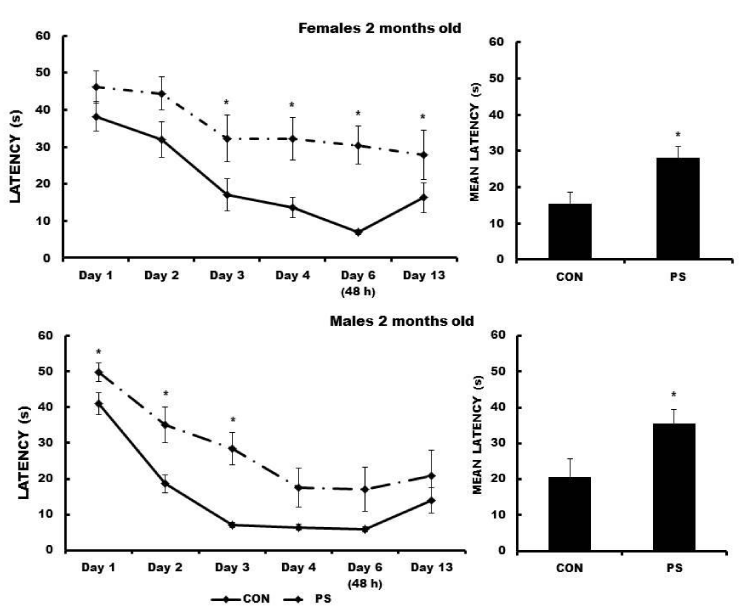
Escape latencies in two-month-old PS females were significantly longer on days 3, 4, 6, and 13 during MWM training (F (5, 44) = 5.55, p < 0.03152). The mean escape latency of PS females was significantly longer as compared to that obtained by the CON group (t = -4.2937, p < 0.000039). The two-month-old PS males showed significantly longer escape latencies as compared to the respective CON males on days 1 through 3 of the MWM training (F (5, 44) = 8.49, p < 0.010142). PS animals swam around the maze most of the time also. As well, the mean escape latency obtained by the PS males was statistically longer than that obtained by the respective CON males (t = -3.9082, p < 0.000164) (Figure 3).
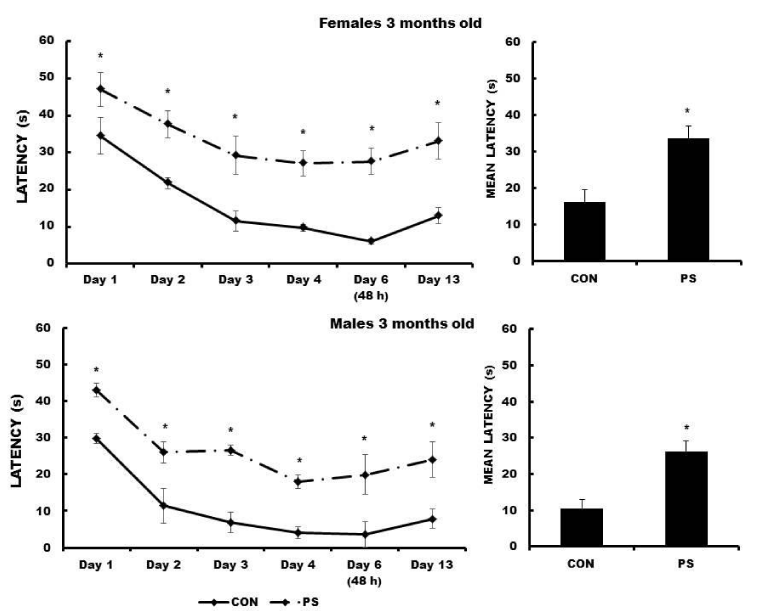
Escape latencies obtained by three-month-old PS females (F (5, 44) = 14.20, p < 0.001407) and males (F (5, 44) = 5.85, p < 0.025740) were significantly longer for all training days as compared to those obtained by the respective CON animals. PS animals swam around the maze most of the time and seem not to look for the platform. Also, mean escape latencies in PS animals of both sexes were significantly longer as compared to those in the respective CON animals (females: t = -5.7410, p < 0.00001; males, t = -3.5122, p < 0.000621) (Figure 4).
The percentage of animals that did not reach the platform in the maze on day 13 of training was analyzed. The percentage of one- (X20.05,1 = 0.6025) and three-months-old (X20.05,1 =8.5714) PS females was significantly larger as compared to the respective CON females. There were not differences between groups of males that did not reach the platform (Table 1).
Prenatal stress effects on time spent in the platform quadrant and platform-site
Crossovers: On day 7, animals performed a 60-second test trial in the MWM without the platform. In these sessions, the time spent in the platform quadrant (NE quadrant) and the number of platform-site crossovers was registered.
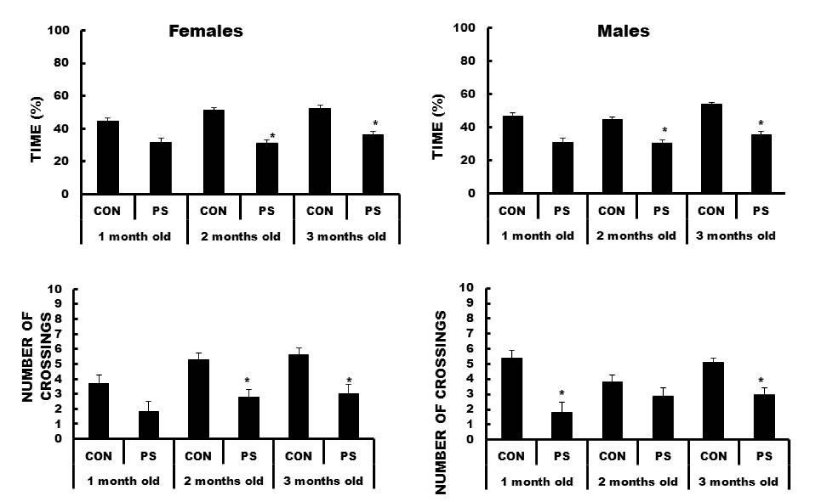
Two- and three-month-old female PS animals spent significant less time in the NE quadrant as compared to the corresponding CON females (F (5, 94) = 4.99, p < 0.001204). Likewise, two- and three-month-old PS males spent shorter time in the NE quadrant (F (5, 94) = 9.24, p < 0.000004).
On the other hand, two- and three-month-old PS females showed less platform-site crossovers as compared to the respective CON females (F (5, 94) = 5.28, p < 0.000810). Two- and three-month-old PS males showed less platform-site crossovers as compared to the corresponding CON males (F (5, 94) = 10.07, p < 0.000001). See Figure 5.
Corticosterone concentrations in female and male offspring
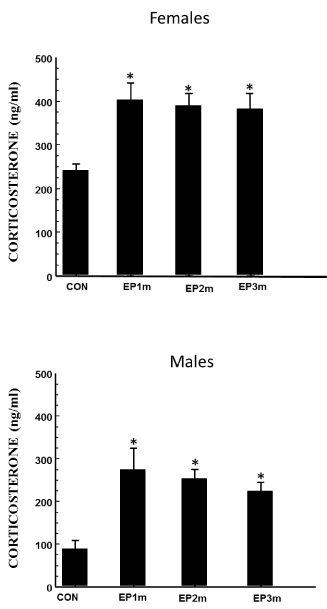
The levels of corticosterone in PS female (F (3, 42) = 11.495, p < 0.00001) and male (F (3, 42) = 8.762, p < 0.0001) offspring at the end of MWM tasks (day 13) were significantly higher than in control offspring at all ages evaluated (Figure 6).
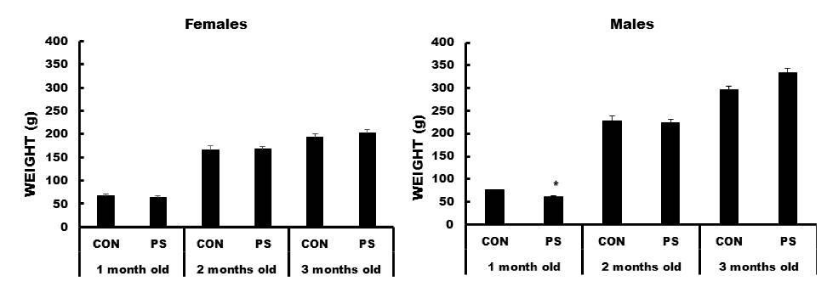
Body Weight: Only the one-month-old PS males showed body weights significantly lower than the CON males (F (5, 94) = 4.80, p < 0.01434). In the two- and three-month old PS males, there were no differences between the body weights of PS and CON. There were not differences in females at any age (Figure 7).
Discussion
The results of this work demonstrate that stress by immersion in cold water causes disruption in learning and memory in offspring during postnatal life. The increase in glucorticoids during pregnancy due to stress can cross the placental barrier as well as fetal blood brain barrier, and can modify the function of fetal brain structures such as brain cortex and the hippocampus [26], and these alterations can disturb behaviors such as learning and memory in the offspring [12,22]. Indeed, the results obtained in this work show that PS has adverse effects on learning and memory during the diverse stages of postnatal life. Longer escape latencies registered in the MWM test, for the PS animals suggest slow learning, as well as retrieval problems. These data indicate that rats PS by immersion in cold water have spatial learning and memory deficiencies in the MWM test independently of their age and sex. PS subjects showed difficulties in finding the platform, and spent most of time swimming around the maze, which indicates that PS rats are not capable of using spatial cues to find the platform. These results differ from previous reports where female and male rats PS stressed by immobilization and bright light [27], or using a stressor battery (immobilization, forced swimming, and elevated maze) [18,20,28], neither PS females nor males showed deficiencies in spatial learning. These differences may be explained by the type of stressor used in this study, due to its larger intensity as compared to other stressors [24]. Other studies report that PS induced by a physical stressor affects one-month-old rats of both sexes, but psychological stressors only affect males [21]. These authors suggest that increased cognitive ability in PS females as compared to that in PS males may be explained by the neuroprotective effect of estrogens in females, which makes females less susceptible to present cognitive disorders. In addition, estrogens increase the number of dendritic spines, long-term potentiation (LTP) and N-methyl-D-aspartate (NMDA) receptors in the hippocampus and influence cholinergic transmission in the forebrain, which promotes learning and memory in females [29,30]. The afore-mentioned supports the hypothesis that estrogens may be protecting brain structures involved in learning and memory of spatial information in PS females when the immobilization technique is used. Furthermore, it has been shown that prenatal stress by immobilization does not alter the estrous cycle of female rats [31] or estrogen levels during adulthood [32]. In this study, PS was induced by immersion in cold water, which is categorized as a high intensity stressor producing lager activation in the adrenal axis as compared to stress by immobilization [33]. We have observed that PS by immersion in cold water may alter the progression of the estrous cycle and diminish the plasmatic concentration of estradiol (data not published). Therefore, the changes in cognitive performance of PS females found in this work may be explained by the exposure to high concentrations of maternal corticosterone observed, alterations in the adrenal axis of the offspring, showing high concentrations of corticosterone during adulthood, as has been reported previously [22], as well as low estrogen concentrations. PS produces hyper activation of the adrenal axis during adulthood because basal concentrations of corticosterone in PS are larger than those observed in non-PS animals [22]. Furthermore, the adrenal axis in PS animals is hyper reactive because of the elevated concentrations of corticosterone as compared to those of non-PS animals before novel and stressful situations [33-36] and such concentrations even take longer to return to basal levels after the exposure to stressors [37]. These alterations in the adrenal axis can importantly contribute to deterioration of spatial learning [38]. The results of this work show that corticosterone levels are significantly higher in PS animals compared with controls. These results are similar to those reported previously in our laboratory showing that three-month-old PS rats of both sexes present deficiencies in spatial learning, and increase in the concentration of serum corticosterone after performing the MWM task [22]. Therefore, cognitive deficiencies in the PS offspring of both sexes in the three ages studied herein may be due, at least in part, to the high concentrations of corticosterone produced by the stress response (adrenal axis hyper - reactivity) resulting from the exposure to the MWM task. Learning deficiencies in the offspring PS by immersion in cold water could also be a consequence of decreased plasticity in the hippocampus of these animals. Additionally, hippocampal CA1 presents a decrease in LTP, which can be apparent until adulthood [39]. These results suggest that PS may deteriorate learning ability during youth and adult stages independently on the sex of the individual.
In this study, the retrieval ability of the PS offspring to perform the MWM task was evaluated. Escape latency and platform-site crossovers were measured 48 hours and one week after the fourth training day. One- and three-month-old, male and female PS rats of the three ages obtained longer escape latencies compared to CON animals 48 hours after training; the same result was obtained 13 days later, except for the one-month-old PS female group. Also, 42.8 % of one-month-old and 60 % of three-month-old PS females could not find the platform at day 13, one week after the learning of the task. Otherwise, PS rats of both sexes spent less time in the platform quadrant and performed less platform-site crossovers as compared to the respective CON rats. These results indicate that PS rats of both sexes present deficiencies in storage and retrieval of the information acquired during training. There are reports that one-month-old PS offspring of both sexes spend less time in the platform quadrant and present less platform-site crossovers [20,40], but this effect is not observed in two-month-old males [18]. There are few reports of follow-up until adulthood in which females are included. In this work, PS female rats also showed deficits in the ability to remember spatial information at the three ages analyzed and in both sexes. Learning and memory differences in PS animals of this work, as compared to other studies, are probably due to the type of stressor used and its intensity. Immobilization is a stressor of mild intensity and its repeated application produces habituation. This mild-intensity stressor, applied in rats, results in a robust initial secretion of corticosterone, which gradually decreases through the days of exposure to the stressor [24,41,42]. The stressor by immersion in cold water does not produce habituation, as demonstrated in a study where rats exposed to this stressor during 20 sequential days presented corticosterone concentrations increased by 3.7 times the normal concentrations, which also remained increased along all the stressor-exposure period [24]. Thus, we propose that high corticosterone concentrations in fetal serum during the last gestational period are produced by the exposure to the stressor by immersion in cold water, also altering brain development, and learning and memory processes. Hence, effects of PS by immersion in cold water on spatial learning and memory in the animals of both sexes studied herein may result from the exposure to high concentrations of corticosterone during fetal development. The hippocampus is a brain structure involved in processing of spatial information and it is one of the main structures targeted by corticosteroids during neonatal brain development. Various studies report that PS animals show diminished dendritic arborization, dendritic-spine density and synapses [43-45]. There are reports of decrease in dendritic segments and arborization of granular and pyramidal cells in the hippocampal CA3 region [46]. Even more, PS produces reduction in cellular proliferation, brain-derived neurotrophic factor (BDNF) [47], neurogenesis, and total hippocampal volume [15,48]. In a previous study, we reported that PS causes decreased neurogenesis in the hippocampus of males but not in females, which correlates with learning and memory deficiencies. This decreased neurogenesis correlates as well with increased corticosterone levels in PS animals [22], which can explain the deficiencies in learning and memory observed in PS females. Therefore, the results of this work can be explained by reduced hippocampal neurogenesis in both females and males
Body weight was lower only in one-month-old PS males, compared to the corresponding CON animals, which were later recovered to the normal. PS females did not show differences at any age. These results are different from other reports where no alteration in body weight were observed in males or females as an effect of PS from day 20 through 80 of postnatal life [19]. Data from this work suggest that PS by immersion in cold water may influence body weight, depending on their sex and age, being one-month-old males the most vulnerable to its effects.
Conclusion
In conclusion, this work shows that prenatal stress induced by immersion in cold water disrupts the learning and memory processing of spatial information in the offspring of both sexes, and these deficiencies persist from infancy to adulthood. These deficiencies could be related, in part, to the high levels of corticosterone observed in male and female offspring. Prenatal stress alters body weight of male rats during childhood, as well.
The authors want to express their gratitude to Dr. Susana Castro Chavira for the translation of the manuscript.
- Takahashi LK (1998) Prenatal stress: consequences of glucocorticoids on hippocampal development and function. Int J Dev Neurosci 16: 199–207. Link: https://goo.gl/TRBHWU
- Takahashi LK, Turner JG, Kalin NH (1998) Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: implications for prenatal stress studies. Psychoneuroendocrinology 23: 571 - 581 Link: https://goo.gl/YhO0wN
- Tuchscherer M, Kanitz E, Ottena W, Tuchscherer A (2002) Effects of prenatal stress on cellular and humoral immune responses in neonatal pigs. Vet Immunol Immunopathol 86: 195 - 203 Link: https://goo.gl/B2fE8f
- Lodge DJ, Grace AA (2011) Developmental pathology, dopamine, stress and schizophrenia. Int J Dev Neurosci 29: 207–213 Link: https://goo.gl/NTFDEh
- Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, et al. (2005) Timing of prenatal stressors and autism. J Autism Dev Disord. 35: 471 - 478 Link: https://goo.gl/yjxb22
- Kinney DK, Munir KM, Crowley DJ, Miller AM (2008) Prenatal stress and risk for autism. Neurosci Biobehav Rev 32: 1519 - 1532 Link: https://goo.gl/ZUe3JF
- Grizenko N, Shayan YR, Ter-Stepanian M (2008) Relation of maternal stress during pregnancy to symptom severity and response to treatment in children with ADHD. J Psychiatry Neurosci 33: 10-16 Link: https://goo.gl/ipjxBh
- Kim HW, Cho SC, Kim BN, Kim JW, Shin MS, et al. (2009) Perinatal and familial risk factors are associated with full syndrome and subthreshold attention-deficit hyperactivity disorder in a korean community sample. Psych Invest 6: 278-285 Link: https://goo.gl/9SYVJE
- Li J, Olsen J, Vestergaard M, Obel C (2010) Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: a nationwide follow-up study in Denmark. Eur Child Adolesc Psychiat 19: 747-753 Link: https://goo.gl/aMRC5P
- King S, Laplante DP (2005) The effects of prenatal maternal stress on children's cognitive development: Project Ice Storm. Stress 8: 35-45 Link: https://goo.gl/PRnHXh
- Laplante DP, Brunet A, Schmitz N, Ciampi A, King S (2008) Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5½-year-old children. J. American Acad Child Adolesc Psychiat 47: 1063-1072 Link: https://goo.gl/WyyMpZ
- Weinstock M (2011) Sex-dependent changes induced by prenatal stress in cortical and hippocampal morphology and behaviour in rats: an update. Stress 14: 604-613 Link: https://goo.gl/5wVjw7
- Wüst S, Entringer S, Federenko IS, Schlotz W, Hellhammer DH (2005) Birth weight is associated with salivary cortisol responses to psychosocial stress in adult life. Psychoneuroendocrinology 30: 591-598 Link: https://goo.gl/4iZNHf
- Weinstock M (2007) Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem Res 32: 1730-1740 Link: https://goo.gl/jrqYWO
- Lemaire V, Koehl M, Le Moal M, Abrous DNV (2000) Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Nat Acad Sci 97: 11032-11037 Link: https://goo.gl/GrJaiX
- Markham JA, Taylor AR, Taylor SB, Bell DB, Koenig JI (2010) Characterization of the cognitive impairments induced by prenatal exposure to stress in the rat. Front Behav Neurosci 4: 278-285 Link: https://goo.gl/hvXeUk
- Zhang Z, Zhang H, Chen Z (2012) Neonatal handling and environmental enrichment increase the expression of GAP-43 in the hippocampus and promote cognitive abilities in prenatally stressed rat offspring. Neurosci Let 522: 1-5 Link: https://goo.gl/skUsRY
- Salomon S, Bejar C, Schorer-Apelbaum D, Weinstock M (2011) Corticosterone mediates some but not other behavioural changes induced by prenatal stress in rats. J Neuroendocrinol 23: 118-128 Link: https://goo.gl/LsJcF5
- Bowman RE, Maclusky NJ, Sarmiento Y (2004) Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology 145: 3778-387 Link: https://goo.gl/wK7u3d
- Zagron G, Weinstock M (2006) Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behav Brain Res 175: 323-328 Link: https://goo.gl/hSQKeU
- Nazeri M, Shabani M, Ghotbi Ravandi S, Aghaei I, Nozari M, et al. (2015) Psychological or physical prenatal stress differentially affects cognition behaviors. Physiol behav 142: 155-160 Link: https://goo.gl/gSk8rQ
- Guerrero Aguilera MA, Rubio MC, Portillo W, Retana-Márquez S (2016) Tactile stimulation effects on hippocampal neurogenesis and spatial learning and memory in prenatally stressed rats. Brain Res Bull 124: 1-11 Link: https://goo.gl/Jq9lMB
- Retana-Márquez S, Domínguez-Salazar E, Velázquez-Moctezuma J (1996) Effect of acute and chronic stress on masculine sexua behavior in the male rat. Psychoneuroendocrinology 21: 39-50 Link: https://goo.gl/lAoZwE
- Retana-Márquez S, Bonilla-Jaime H, Vázquez-Palacios G, Domínguez-Salazar E, Martínez-García R, et al. (2003) Body weight gain and diurnal differences of corticosterone changes in response to acute and chronic stress in rats. Psychoneuroendocrinology 28: 207-227 Link: https://goo.gl/hM5aPU
- Kapoor A, Kostali A, Janus C, Matthews SG (2009) The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav. Brain Res 197 144-149 Link: https://goo.gl/8pd48h
- Charil A, Laplante DP, Vaillancourt C, King S (2010) Prenatal stress and brain development. Brain Res Rev 65: 56-79 Link: https://goo.gl/dX2ZRw
- Zuena AR, Mairesse J, Casolini P, Cinque C, Alema GS, et al. (2008) Prenatal Restraint Stress Generates Two Distinct Behavioral and Neurochemical Profiles in Male and Female Rats. PLoS ONE 3: 1–13 Link: https://goo.gl/mmO0Nz
- Yaka R, Salomon S, Matzner H, Weinstock MR (2007) Effect of varied gestational stress on acquisition of spatial memory, hippocampal LTP and synaptic proteins in juvenile male rats. Behav Brain Res 179: 126-132 Link: https://goo.gl/rh1Jha
- McEwen BS, Alves SE, Bulloch K, Weiland NG (1997) Ovarian steroids and the brain implications for cognition and aging. Neurology 48: 8S – 15. Link: https://goo.gl/3keJi5
- Tanapat P, Hastings NB, Reeves AJ, Gould E (1999) Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci 19: 5792 – 5801. Link: https://goo.gl/oLBxm0
- Beckhardt S, Ward IL (1983) Reproductive functioning in the prenatally stressed female rat. Dev Psychobiol 16: 111 - 118. Link: https://goo.gl/6ScTet
- Walf AA, Frye CA (2007) Estradiol decreases anxiety behavior and enhances inhibitory avoidance and gestational stress produces opposite effects: Research Report. Stress 10: 251 – 260. Link: https://goo.gl/VakM4V
- de Vries A, Holmes MC, Heijnis A, Seier JV, Heerden J, et al. (2007) Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest 117: 1058 - 1067 Link: https://goo.gl/gtxOUC
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR (1996) Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology 64: 412 - 418 Link: https://goo.gl/aK9zuc
- Welberg LA, Seckl JR, Holmes MC (2000) Inhibition of 11??hydroxysteroid dehydrogenase, the foeto?placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety?like behaviour in the offspring. Eur J Neurosci 12: 1047 – 1054. Link: https://goo.gl/KGXe8z
- Welberg LA, Seckl JR (2001) Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol 13: 113 – 128. Link: https://goo.gl/PkPp7r
- Koehl M, Darnaudéry M, Dulluc J, Van Reeth O, Le Moal M, et al. (1999) Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol 40: 302 - 315 Link: https://goo.gl/ZKo8DY
- Schwabe L, Schächinger H, de Kloet ER, Oitzl MS (2010) Stress impairs spatial but not early stimulus-response learning. Behav Brain Res 213: 50 - 55 Link: https://goo.gl/TOcIW6
- Son GH, Geum D, Chung S, Kim EJ, Jo JH, et al. (2006) Maternal stress produces learning deficits associated with impairment of NMDA receptor-mediated synaptic plasticity. J Neurosci 26: 3309 – 3318. Link: https://goo.gl/CVX4C7
- Yang J, Han H, Cao J, Li L, Xu L (2006) Prenatal stress modifies hippocampal synaptic plasticity and spatial learning in young rat offspring. Hippocampus 16: 431 - 436 Link: https://goo.gl/ph8IC2
- Natelson BH, Ottenweller JE, Cook JA, Pitman D, McCarty R, et al. (1988) Effec of stressor intensity on habituation of the adrenocortical stress response. Physiol Behav 43: 41 – 46. Link: https://goo.gl/0W0aqs
- Pitman DL, Ottenweller JE, Natelson BH (1988) Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiol Behav 43: 47 – 55. Link: https://goo.gl/6EiLW6
- Hayashi A, Nagaoka M, Yamada K, Ichitani Y, Miake Y, et al. (1998) Maternal stress induces synaptic loss and developmental disabilities of offspring. Int J Dev Neurosci 16: 209 - 216. Link: https://goo.gl/atTOBi
- Ishiwata H, Shiga, T, Okado N (2005) Selective serotonin reuptake inhibitor treatment of early postnatal mice reverses their prenatal stress-induced brain dysfunction. Neuroscience 133: 893 – 901. Link: https://goo.gl/5OPjZ5
- Barros VG, Duhalde-Vega M, Caltana L, Brusco A, Antonelli M C (2006) Astrocyte-neuron vulnerability to prenatal stress in the adult rat brain. J Neurosci. Res 83: 787 - 800 Link: https://goo.gl/8Rrxi9
- Hosseini-sharifabad M, Hadinedoushan H (2007) Prenatal stress induces learning deficits and is associated with a decrease in granules and CA3 cell dendritic tree size in rat hippocampus. Anat Sci Int 82: 211 – 217. Link: https://goo.gl/MqDJbC
- Van den Hove DL, Steinbusch HW, Bruschettini M, Gazzolo D, Frulio R, et al. (2006) Prenatal stress reduces S100B in the neonatal rat hippocampus. Neuroreport 17: 1077 - 1080 Link: https://goo.gl/CLvOyF
- Coe CL, Kramer M, Czéh B, Gould E, Reeves AJ, et al. (2003) Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiat 54: 1025 – 1034. Link: https://goo.gl/1B3blY
- Nishio H, Kasuga S, Ushijima M, Harada H (2001) Prenatal stress and postnatal development of neonatal rats-sex-dependent effects on emotional behavior and learning ability of neonatal rats. Int J Dev Neurosci 19: 37 – 45. Link: https://goo.gl/QG8dKi
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
