Assessment of Chromium Oxide Nanoparticles Intake in Rattus norvegicus by Primary Renal Function Markers and RBC Architecture
Section of Genetics, Department of Zoology Aligarh Muslim University, Aligarh
Author and article information
Cite this as
Fatima R, Ahmad R (2017) Assessment of Chromium Oxide Nanoparticles Intake in Rattus norvegicus by Primary Renal Function Markers and RBC Architecture. Glob J Zool. 2017; 2(1): 008-012. Available from: 10.17352/gjz.000005
Copyright License
© 2017 de los Angeles GAM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Haematological tests are signifi cant diagnostic tools that are equally valuable as indicators of toxic insult or stress due to xenobiotics and environmental fl uctuations. Present study was designed to investigate alterations in primary renal function markers, pathological changes in kidneys and variations in RBCs shape of male wistar rats due to chromium oxide nanoparticles (Cr2O3NPs) exposure. Cr2O3NPs are transition metal oxide NPs which are widely being used as catalysts, pigments and coating materials. Therefore, toxicological evaluation is fundamental with respect to their increasing applications. In the current study, synthesis of Cr2O3 NPs was accomplished by sol - gel method and characterized in sequential manner by electron microscopy (TEM and SEM). TEM analysis revealed size - distribution of test NPs in the range 22.50 ± 1.76 nm. SEM represented the morphological features with high homogeneity of sample NPs validating Cr2O3NPs synthesis. Toxicological fi ndings revealed deviations in renal function test of treated rats with respect to the control group indicative of kidney damage. Blood Urea Nitrogen (BUN) was found to be signifi cantly higher (p < 0.05) after 14 days high dose exposure in comparison to control rats. Extensive changes in kidneys architecture were noted after repeated exposures to high dose. Various structural deformations of RBCs including tear drop cells, bite cells, elliptocytes, echinocytes etc were also observed. Results of present investigations, though preliminary but clearly demonstrate that oral administration of Cr2O3 NPs induces biochemical changes consequently leading to alterations in renal function parameters and RBC shapes of exposed rats.
Nanotechnology is a fast - growing research field that has led to many significant scientific breakthroughs [1]. It involves the formation and manipulation of particles at ‘nanoscale’ having novel and improved physico - chemical properties [2-4]. Owing to the minuscule magnitudes, nanoparticles (NPs) acquire unique physical, chemical, electrical and magnetic properties. The exclusive characteristics have resulted in increasing synthesis and widespread usage of engineered NPs. Prevalent applications of NPs have thereby conferred them with enormous toxic potential on human health and environment. The NPs being very small in size can pervade the cellular membrane and interfere in the cell’s natural processes [5,6]. Entry of NPs via various routes like oral, dermal and inhalation may occur either intentionally or unintentionally, resulting in subjugating normal cell functioning [7, 8]. So, safety concerns have arisen regarding the risk assessment of manufactured NPs. Blood plays an integrated and inevitable part of the biological system and haematology can visibly ascertain the diseased or stressed state of animals [9-11]. Many workers have studied effects of several heavy metals on haematological parameters in various model organisms [12-15]. Gardner and Yeuich [16] have established long - term effects of heavy metal on blood chemistry parameters. Jawad et al. [17], advocated that haematological parameters may serve as early biomarkers of toxicity. A study by Ahmad et al. [18], established the correlation between NDMA - induced hepatic fibrosis and changes in clinical blood parameters of wistar rats. Likewise, serum biochemical indices also play a significant role in monitoring clinical symptoms produced by a toxicant [19]. However, research on haemato - toxic effects due to NPs exposure are limited and such study concerning ‘nano’ Cr2O3 are lacking. Cr2O3 NPs are industrially important which are being widely used as pigments, catalysts, for thermal and wear protection coating, sensing of humidity and in many other applications [20-23]. Owing to the development and applications of Cr2O3 NPs, it is important to cautiously evaluate their toxicity. Well-designed experiments with a consistent approach and reasonable interpretations are required to determine the risks associated with nanomaterials. In view of the present literature, attempts are made to investigate the effect of Cr2O3 NPs taking red blood cells (RBCs) phenotype and renal function profile as indicators of toxicity in wistar rats. These tests are used in examining the response of an organism to toxic insult and can be efficiently employed for toxicity evaluation of NPs. For this study, rats were exposed to two concentrations of Cr2O3 NPs for multiple durations to study discrepancies in renal function profile and changes in shape of RBCs due to acute or cumulative dosing. The study may help in curbing nanotoxicity at initial stages by monitoring these parameters in exposed organisms.
Methodology
Preparation and characterization of Cr2O3 NPs
Cr2O3 NPs were prepared by sol - gel method followed by characterization using transmission electron microscopy (TEM) and scanning electron microscopy (SEM). For size distribution analysis, TEM of Cr2O3 NPs aqueous solution was performed on JEOL 100/120 kV transmission electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 120 kV. For the morphological analysis, SEM was done using fine Cr2O3 NPs powder on a carbon tape in a JSM-6510LV scanning electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of ~ 20 kV.
Preparation and characterization of Cr2O3 NPs
Cr2O3 NPs were prepared by sol - gel method followed by characterization using transmission electron microscopy (TEM) and scanning electron microscopy (SEM). For size distribution analysis, TEM of Cr2O3 NPs aqueous solution was performed on JEOL 100/120 kV transmission electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of 120 kV. For the morphological analysis, SEM was done using fine Cr2O3 NPs powder on a carbon tape in a JSM-6510LV scanning electron microscope (JEOL, Tokyo, Japan) at an accelerating voltage of ~ 20 kV.
Animals and ethical approval
Male albino wistar rats (Rattus norvegicus) aged 7 - 8 weeks and weighing 145 ± 10 gm were procured from the Central Animal House, Jawaharlal Nehru Medical College (JNMC), AMU. The animals were housed in polycarbonate cages in a hygienic condition at a temperature of 22 ± 3°C and relative humidity of 55 - 65%, on a 12 / 12 hours light / dark cycle. Commercially available sterilized food pellets and quality drinking water was offered ad libitum. Care, handling and sacrifice of animals were in accordance with the guidelines of Institutional Animal Ethics Committee (IAEC; No: 401/RO/C/2001/CPCSEA); CPCSEA, India.
Sample NPs and experimental design
Normal control and treated groups were randomly allocated with 5 animals each and acclimatized for a week. Cr2O3 NPs were suspended in double distilled water and in order to avoid the aggregation, stock suspensions were stirred on vortex agitator for 5 min, each day prior to dosing. The concentrations of Cr2O3 NPs in stock suspensions were 50 µg / ml and 200 µg / ml, respectively. Each dose of Cr2O3 NPs [50 µg / 100 gm bwt (low dose) and 200 µg / 100 gm bwt (high dose)] was administered orally in wistar rats for multiple durations i.e. 24 hours (single dose), once daily for 7 days and for 14 consecutive days, respectively. Haematological parameters were monitored in control and exposed rats. To investigate renal function enzymes and their changes in response to Cr2O3 NPs exposure, normal range of these factors were initially measured in serum of control rats. The control group received equivalent volume of the vehicle (double distilled water) for the same number of days.
Blood sampling
Whole blood sample was collected at the end of each dose - duration exposure. 5 ml of blood sample was withdrawn from the heart of each rat by direct cardiac puncture through sterilized syringe into EDTA vials and clot activator vials for RBC shape and serum study, respectively. The blood samples drawn from animals were used for preparing permanent smears of RBCs. For observation of deformation in RBCs shape, slides were fixed in methanol for 12 - 15 min, stained with Giemsa for 8 - 10 min and fixed in DPX. The stained slides were then randomly selected for rheological studies and observed on Nikon ECLIPSE E200 microscope at 40 X magnification. For renal function test, serum was separated by centrifugation at 3000 rpm for 10 min and kept at - 80°C until used for biochemical estimations. Activities of kidney function (BUN i.e. blood urea nitrogen, uric acid, creatinine, Na+, K+) were measured in the serum of rats by an automatic biochemical analyzer (Cobas Mira Plus, Roch Diagnsotics, Germany).
Data and statistical analysis
All observations were replicated thrice for varied observations and the data are expressed as mean ± SEM. Statistical analyses were performed using Graph Pad Prism software version 3.02. Groups’ variance was analyzed by one way analysis of variance (ANOVA) followed by post hoc Tukey to test for significant difference between the groups. The level of significance was set as p < 0.05 (*).
Results
Preparation and characterization
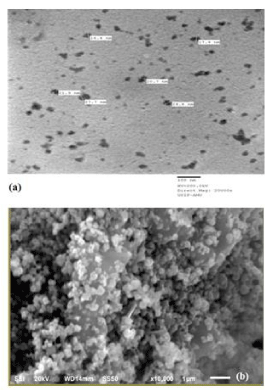
The size - distribution of Cr2O3 NPs was determined to be 22.50 ± 1.76 nm in diameter by TEM, illustrated in Figure 1(a). SEM observations revealed high homogeneity of the sample and showed micrometric aggregate formation consisting of nanometric spherical particles (Figure 1b), validating successful synthesis of Cr2O3 NPs.
RBC Shape
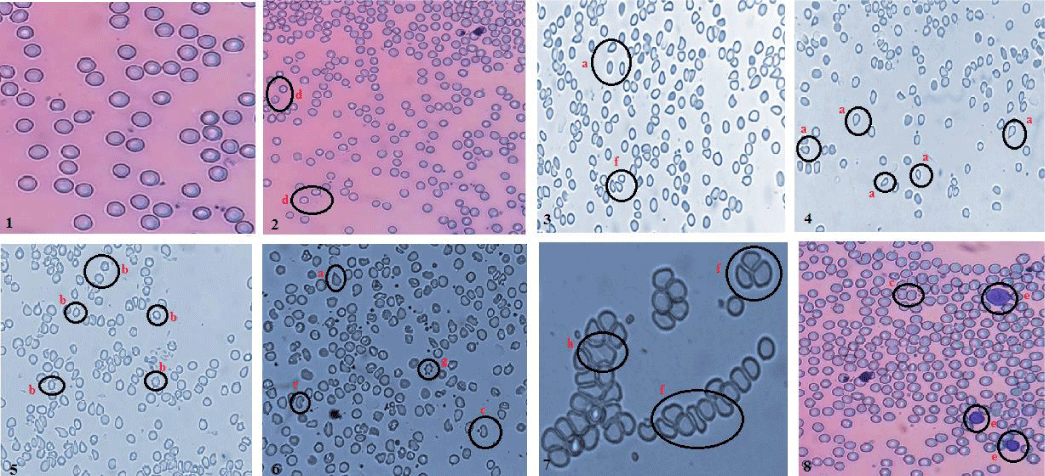
Although quantitative assessment was not performed but various deformities in shape of RBCs like tear drop red cells, acanthocytes, echinocytes, bite cells, elliptocytes and spherocytes were observed extensively in exposed rats. These structural distortions in RBCs were noted to increase with dose - duration exposure of Cr2O3 NPs (Figure 2).
Renal function markers
Serum levels of various biomolecules are frequently monitored in diagnosing various diseases. Results of this study clearly demonstrate infliction of toxicity following Cr2O3 NPs exposure consequently leading to renal injury. It is evident from the alterations in kidney function markers noticed in serum of test groups at both doses and all duration exposure of Cr2O3 NPs, as compared with control group (Tables 1,2). There was rise in serum creatinine and uric acid levels noted at both doses after 14 days of repeated exposure but, compared to control group, their elevated levels were insignificant. However, in case of BUN, significant increase was observed for high dose repeated exposure (14 days, p < 0.05). No apparent variations in treated groups were observed for Na+ and K+ levels when compared to control group. But, significant elevation in Na+ level (p < 0.05) was reported after 14 days exposure to high dose in comparison to low dose indicating inefficiency of kidneys in rats due to continual insult of NPs stress. However, animals with elevated renal function markers did not show any noticeable clinical signs. In addition, kidneys were also examined for histological changes after repeated (7 days and 14 days) high dose administration of Cr2O3 NPs. It was clearly observed that prolonged exposures of Cr2O3 NPs significantly changed the architecture of kidneys in treated groups when compared with controls. Congested glomeruli and focal tubular atrophy was noticed after 7 days exposure. After 14 days exposure congested interstitial tissues, marked vacuolization of tubular cells and inflammation were noticed. The tested concentrations of Cr2O3 NPs caused renal damage, prominently with durations indicating kidneys to be one of the major target organs.
Discussion
Nanotechnology research warrants toxicological examination of NPs due to their increasing use and synthesis. Assessment of serum biochemical parameters and shape of RBCs definitely assures feasible and early detection of response of an organism to any stressor in the body or environment. This fact was reasonably and logically employed in the present study for toxicity evaluation of Cr2O3 NPs in rats with special focus on primary renal markers and RBCs shape. In the current study, Cr2O3 NPs were successfully synthesized by sol - gel method and primarily characterized by electron microscopy. SEM and TEM observations clearly demonstrated Cr2O3 NPs to be well dispersed displaying average particle size of 22.50 ± 1.76 nm. For toxicological evaluation of Cr2O3 NPs exposure, discrepancies in established renal function markers activities in serum of rats were noted as they effectively display damage or harm to the organ. The kidneys are supposed to be highly vascular organ and play an important role in detoxification as well as many metabolic processes. They have a host of functional enzymes and any disturbance or injury to the renal tissues would thereby affect the normal level of measurable biochemical parameters of this organ in blood. When kidney cells are inflamed or damaged due to toxicity or diseases, these markers spill into bloodstream leading to a rise in their levels. Therefore, anomalous levels of associated biochemical parameters in serum signify injury to the organs leading either to adaptive response or ultimately to severe damage after continual exposure. Thus, in our study, aberrant expression in renal function profile (BUN, creatinine, uric acid, Na+ and K+ levels) of treated rats clearly pinpoints injury to the kidney of treated rats as compared to control rats. This study shows that functional activity of kidneys happens to be disturbed due to Cr2O3 NPs exposure which resulted in the leakage of some of the investigated renal parameters. In general, renal function biomolecules have been reported to spill over into the serum upon exposure to toxic materials [24-26]. Furthermore, it can be hypothesized that Cr2O3 NPs exposure may lead to dysfunction of renal haemopoiesis as the kidney function is found to be impaired in treated rats. Considering the biological clearance function of kidneys, deviations of serum parameters from normal control was well anticipated. Multiplicity of structural deformations observed in RBCs of rats might be due to stress of NPs exposure and may indicate towards onset of apoptosis which is a matter of critical concern. These findings clearly signify that after oral administration, Cr2O3 NPs get efficiently absorbed in the digestive tract and ultimately released into blood as evidenced by deformed RBCs and altered renal profile. Some previous studies have also indicated that nano-sized particles may possibly cross the small intestine by persorption and further distribute into blood, brain, lungs, heart, kidneys, spleen, liver, intestine and stomach [27, 28]. NPs deposition in vital organs or tissues could induce cellular damage [29]. It is generally agreed that upon ingestion NPs can be absorbed and that absorption increases with decreasing particle size [30].Structural changes observed in renal tissues are indicative of kidneys being the main target organs of Cr2O3 NPs toxicity that may have occurred through the absorption of these NPs from digestive tract and their subsequent circulation in blood. Obviously possibilities of detrimental effects to other organs cannot be ignored, once NPs come into circulation, but this study is limited to investigate the effect of Cr2O3 NPs on kidneys only. Thus, we can say that administered Cr2O3 NPs deposited in kidneys may have induced cellular damage via ROS formation which resulted in leakage of investigated parameters into the bloodstream [31-33]. The present results taken together strongly indicate kidney damage as well as variations in RBCs shape that may further enhance other metabolic disturbances and cause disease. This study is preliminary on potential haemato - toxicological changes on oral intake of Cr2O3 NPs that may serve as useful information to determine the safety regulations against Cr2O3 NPs toxicity. Future studies are warranted to explore the exact molecular mechanism of this renal toxicity as well as damage to other tissue/organ types.
Conclusions
In conclusion, variations in RBCs shape and alterations of renal function markers are a consequence of Cr2O3 NPs induced toxicity in rats. This investigation may be helpful to monitor and regulate Cr2O3 NPs toxicology by accumulating information on the type of toxicities generated by these NPs in vitro or in vivo. Development of friendly synthetic processes for NPs, such as green synthesis, may be a possible alternative and hope in regressing NP induced-toxicity. From this experimental study, it is suggested that primary toxic potential of Cr2O3 NPs shall be assessed before its use in various industrial sectors using blood and renal function parameters.
The authors are thankful to the Chairman, Department of Zoology; Centre of Excellence in Materials Science (Nanomaterials), Department of Applied Physics; University Sophisticated Instruments Facility (USIF) of Aligarh Muslim University, Aligarh for providing necessary facilities. This work is an extended version of the efforts initiated by the first author under the guidance of (Late) Prof. Anjum Ara. RF gratefully acknowledges the University Grants Commission (UGC), New Delhi, India for the award of MANF fellowship No. F1-17.1/2012-13/MANF-2012-13-MUS-UTT-9927/ (SA-III/Website).
- Aitken RJ, Creely KS, Tran CL (2004) Nanoparticles: An occupational hygiene review. Health and Safety Executive Research Report 274. HSE Books, Norwich, UK. Link: https://goo.gl/1ZfQ0B
- Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP (1998) Semiconductor nanocrystals as fluorescent biological labels. Sci 281: 2013-2016. Link: https://goo.gl/VraAM7
- Taton TA, Mirkin CA, Letsinger RL (2000) Scanometric DNA array detection with nanoparticle probes. Sci 289:1757-1760. Link: https://goo.gl/V2Umor
- Cui Y, Wei Q, Park H, Lieber C (2001) Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Sci 293:1289-1292. Link: https://goo.gl/9NOkG1
- Chen D, Xi T, Bai J (2007) Biological effects induced by nanosilver particles: in vivo study. Biomed Mater. 2:S126-128. Link: https://goo.gl/UB1alZ
- Razavian MH, Masaimanesh M (2015) Ingestion of silver nanoparticles leads to changes in blood parameters. Nanomed J1:339-345. Link: https://goo.gl/Kguo9P
- Peter HH, Irene BH, Oleg VS (2004) Nanoparticles-known and unknown health risks. J Nanobiotechnol 2:12 Link: https://goo.gl/bWNaRJ
- Chen Z , Meng H , Xing G , Chen C , Zhao Y , et al. (2006) Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett 163:109-120. Link: https://goo.gl/Fxxi8S
- Calabrese AL, Thurberg FP, Dawson MA, Wenzloff DR (1975) Sublethal physiological stress induced by cadmium and mercury in white flounder, pseudopleuronectes americanus. In Sublethal effects of toxic chemicals on aquatic animals (edt. Koeman JH and Strik JJTWT) Scientific co. Amsterdam. Elsevier: 15-21. Link: https://goo.gl/WvoI7K
- Hymavathi V, Rao LM (2000) Effect of sublethal concentrations of lead on the haematology and biochemical constituents of Channa punctatus. Bull Pure & App Sci 19: 1-5.
- Siakpere K, Ubogu O, Oghoghene E (2008) Sublethal haematological effects of zinc on the freshwater fish, Heteroclarias sp. (Osteichthyes: Clariidae). African Journal of Biotechnol 7:2068-2073. Link: https://goo.gl/hW13sk
- Singh MP (1995) Hematological responses in a freshwater teleost, Channa punctatus to experimental copper and chromium poisoning. J Environ Biol 16: 339- 341.
- Nanda P, Behera MK (1996) Nickel induced changes in some hemato-biochemical parameters of a cat fish Heteropneustes fossilis (Bloch) 14: 82-85.
- Ray D, Banerjee SK (1998) Hematological and histopathological changes in Clarias batrachus (Linn) exposed to Nickel and Vanadium. Env and Ecol 16: 151-156.
- Vijayamohanan G, Nair A, Suryanarayanan H (2000) Impact of effluent from a TiO2 factory on the peripheral haematology of Oreochromis mossambicus (Peters). J Environ Biol 21: 293-296.
- Gardner GR, Yeuich PP (1969) Studies on the blood morphology of three estuarine cyprinidontiform fishes. J fish Res Bd Canada 26: 433-447. Link: https://goo.gl/o5evpm
- Jawad LA, Al-Mukhtar MA, Ahmed HK (2004) The relationship between haematocrit and some biological parameters of the Indian shad, Tenualosa ilisha (Family: Cyprinidae). Animal Biodiversity and Conservation 27: 47-52. Link: https://goo.gl/ps6WRV
- Ahmad A, Fatima R, Maheshwari V, Ahmad R (2011) Effect of N'-nitrosodimethylamine on red blood cell rheology and proteomic profiles of brain in male albino rats. Interdiscip Toxicol. 4: 125-131. Link: https://goo.gl/ekJfyT
- Adeyemi OS, Akanji MA (2011) Biochemical changes in the kidney and liver of rats following administration of ethanolic extract of Psidium guajava leaves. Human and Experimental Toxicol 30: 1266-1274. Link: https://goo.gl/7vv5uG
- Gibot P, Vidal L (2010) Original synthesis of chromium (III) oxide nanoparticles. J Eur Ceram Soc 30:911-915. Link: https://goo.gl/iCDTHs
- Yang X, Peng X, Xu C, Wang F (2009) Electrochemical Assembly of Ni – xCr – yAl Nanocomposites with Excellent High-Temperature Oxidation Resistance. J Electrochem Soc 156: C167-C175. Link: https://goo.gl/doxsc8
- Makhlouf SA, Bakra ZH, Al-attara H, Moustafaa MS (2013) Structural, morphological and electrical properties of Cr 2 O 3 nanoparticles. Mater Sci Eng 178: 337-343. Link: https://goo.gl/y6eIUK
- Landau MV, Shter GE, Titelman L, Gelman V, Rotter H, et al. (2006) Alumina Foam Coated with Nanostructured Chromia Aerogel: Efficient Catalytic Material for Complete Combustion of Chlorinated VOC. Ind Eng Chem Res 45: 7462-7469. Link: https://goo.gl/IW4D0E
- Perrone RD, Madias NE, Levey AS (1992) Serum Creatinine as an index of renal function; New insight into old concept. Clin Chem 38:1933-1953. Link: https://goo.gl/QqG0zD
- Nerbert WT (1983) Fundamental of clinical chemistry. In: Burtis CA, Ashwood ER, editors. Tietz Texbook of Clinical Chemistry. 3rd ed. Philadelphia: W.B. Saunders: 1056-1092.
- Nkosi CZ, Opoku AR, Terblanche SE (2005) Effect of pumpkin seed (Cucurbita pepo) protein isolate on the activity levels of certain plasma enzymes in CCl4 induced liver injury in low protein fed rats. Phytother Res 19:341-345. Link: https://goo.gl/rYrGfa
- Lankveld DP, Oomen AG, Krystek P, Neigh A, Troost-de Jong A, et al.(2010) The Kinetics of the Tissue Distribution of Silver Nanoparticles of Different Sizes. Biomat 31: 8350-8361. Link: https://goo.gl/0efvA9
- Park K, Park EJ, Chun IK, Choi K, Lee SH, et al. (2011) Bioavailability and Toxicokinetics of Citrate-Coated Silver Nanoparticles in Rats. Arch Pharmacol Res 34: 153-158. Link: https://goo.gl/Sbvw99
- Gatti AM (2004) Biocompatibility of micro- and nano-particles in the colon. Part II. Biomat 25: 385-392. Link: https://goo.gl/POu1OD
- Florence AT (2005) Nanoparticle Uptake by the Oral Route: Fulfilling Its Potential? Drug Discovery Today Technologies 2: 75-81. Link: https://goo.gl/l0FCus
- Fubini B, Hubbard A (2003) Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic Biol Med 34: 1507-1516. Link: https://goo.gl/vruyTd
- Li N, Xia T, Nel AE (2008) The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radic Biol Med 44:1689-1699. Link: https://goo.gl/Hv8Txx
- Huang Y, Wu C, Aronstam R (2010) Toxicity of transition metal oxide nanoparticles: recent insights fromin vitro Studies. Materials 3: 4842-4859. Link: https://goo.gl/JZ3HxL
Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.


 Save to Mendeley
Save to Mendeley
